The Step-by-Step Process of Making Thin-Wall Nitinol Tubing for Microcatheters

Manufacturers create thin-wall nitinol tubing for microcatheter applications using a high-precision, multi-step manufacturing process. This process starts with extrusion, which shapes the tubing to achieve ultra-thin walls and consistent diameters essential for advanced catheters. High-precision extrusion and subsequent steps ensure the tubing reaches a relative density of up to 99%, free of cracks and defects. Tubing must withstand over 10 million cycles of stress, maintain tensile strength between 500 MPa and 900 MPa, and resist corrosion for reliable applications in catheters. Advanced manufacturing techniques drive innovation, enabling catheters to perform in demanding cardiovascular applications and minimally invasive procedures.
The manufacturing process for thin-wall nitinol tubing for microcatheter applications demands strict control at every stage to deliver the superelasticity, biocompatibility, and durability required for next-generation catheters.
Key Takeaways
Thin-wall nitinol tubing combines nickel and titanium to create flexible, strong, and biocompatible tubes essential for advanced microcatheters.
Precise extrusion and multi-stage drawing processes shape the tubing with ultra-thin walls and tight tolerances, ensuring durability and consistent performance.
Heat treatment improves the tubing’s superelasticity and shape memory, allowing it to bend and return to its original form without damage.
Surface finishing and thorough cleaning reduce defects and improve biocompatibility, making the tubing safe for medical use.
Strict quality control and real-time monitoring guarantee the tubing meets high standards for strength, fatigue resistance, and safety in minimally invasive procedures.
Material Selection for Nitinol Tubing
Alloy Composition
Selecting the right alloy composition forms the foundation for high-performance nitinol tubing in microcatheter applications. Manufacturers combine nickel and titanium in precise ratios, typically around 55% nickel and 45% titanium, to achieve the desired properties. This specific blend gives nitinol tubing its unique superelasticity and shape memory, which are essential for devices that must flex and return to their original form. Nitinol tubing with this composition demonstrates excellent fatigue resistance, allowing it to endure repeated bending without permanent deformation.
Uniaxial tension tests, following ASTM F2516 standards, confirm that nitinol tubing maintains its mechanical performance and shape memory even after extensive use. Clinical trials using ASTM F2063 compliant nitinol tubing, such as the Wistend microstent, show outstanding biocompatibility and safety, with minimal tissue reactions and sustained therapeutic effects over six months.
The alloy composition also directly impacts biocompatibility. Nitinol tubing with the correct nickel-titanium ratio reduces the risk of adverse reactions in the body. Corrosion resistance tests reveal that electropolished nitinol tubing surfaces release fewer nickel ions, further enhancing biocompatibility and durability. Manufacturers can tune the elastic modulus of nitinol tubing by adjusting alloy elements, which helps reduce stress shielding and improve implant longevity.
Purity and Quality
High-purity nickel and titanium are critical for producing nitinol tubing that meets strict medical standards. Impurities can compromise the superelasticity and biocompatibility of nitinol tubing, leading to reduced performance and increased risk of failure. Comparative studies show that production methods, such as classical casting and continuous casting, influence the purity and microstructure of nitinol tubing. Continuous casting often results in a better microstructure, which supports superior biocompatibility and mechanical properties.
Reducing grain sizes below 50-100 nm and using advanced alloying techniques can improve the tensile strength of nitinol tubing by up to 35%.
Fatigue resistance can increase by more than 50% through mechanical attrition and nanostructuring.
Corrosion resistance improvements over 90% are possible by incorporating advanced alloying elements and surface treatments.
Nitinol tubing with high purity and optimized composition achieves cell viability rates above 95%, osteointegration improvements up to 40%, and bone ingrowth rates over 80%. These metrics highlight the importance of purity and quality in ensuring the biocompatibility and long-term success of nitinol tubing in microcatheter applications.
Extrusion and Tube Formation

Extrusion Process
The extrusion process stands at the core of manufacturing ultra-thin nitinol tubing for microcatheter applications. Engineers begin by heating nitinol billets above 800°C, which refines the grain structure and enhances mechanical properties. This high-temperature environment allows the nitinol to flow smoothly through a die, forming the initial tube shape. Microextrusion techniques enable the creation of thin walled tubes with diameters as small as 0.2 mm and wall thicknesses below 0.1 mm. The extrusion process must maintain precise control over temperature and deformation to prevent defects such as cracking or uneven surfaces.
Manufacturers rely on advanced process metrics to ensure the effectiveness of extrusion. Dimensional accuracy is critical, especially for seamless tube drawing. For example, outer diameter tolerances can reach ±0.005 mm for tubes under 0.3 mm in diameter. The following table summarizes the tight tolerances achieved during extrusion:
Outer Diameter Range (mm) | OD Tolerance (mm) | ID Tolerance (mm) |
|---|---|---|
≤0.3 | ±0.005 | ±0.010 |
0.3 to 0.5 | ±0.007 | ±0.015 |
0.5 to 1.5 | ±0.015 | ±0.020 |
1.5 to 2.5 | ±0.020 | ±0.030 |
2.5 to 3.5 | ±0.020 | ±0.040 |
Process engineers use non-destructive testing methods, such as ultrasonic and eddy current testing, to detect internal and surface defects without damaging the tubing. Laser micrometers and coordinate measuring machines verify dimensional accuracy within microns. Cold working control during extrusion further enhances the strength and flexibility of nitinol tubing, ensuring the superelasticity required for microcatheter performance.
Precise numerical controls play a vital role in maintaining small diameters and thin walls during extrusion. Operators adjust screw speed, puller speed, and the ratio of puller speed to flow rate to stabilize tube dimensions. For instance, increasing puller speed from 5.3 to 9.3 m/min reduces wall thickness from 0.197 mm to 0.13 mm. Optimized tip and die designs ensure uniform velocity profiles and allow for wall thicknesses as low as 150 µm. The following chart illustrates the relationship between outer and inner diameter tolerances for ultra-thin nitinol tubing:
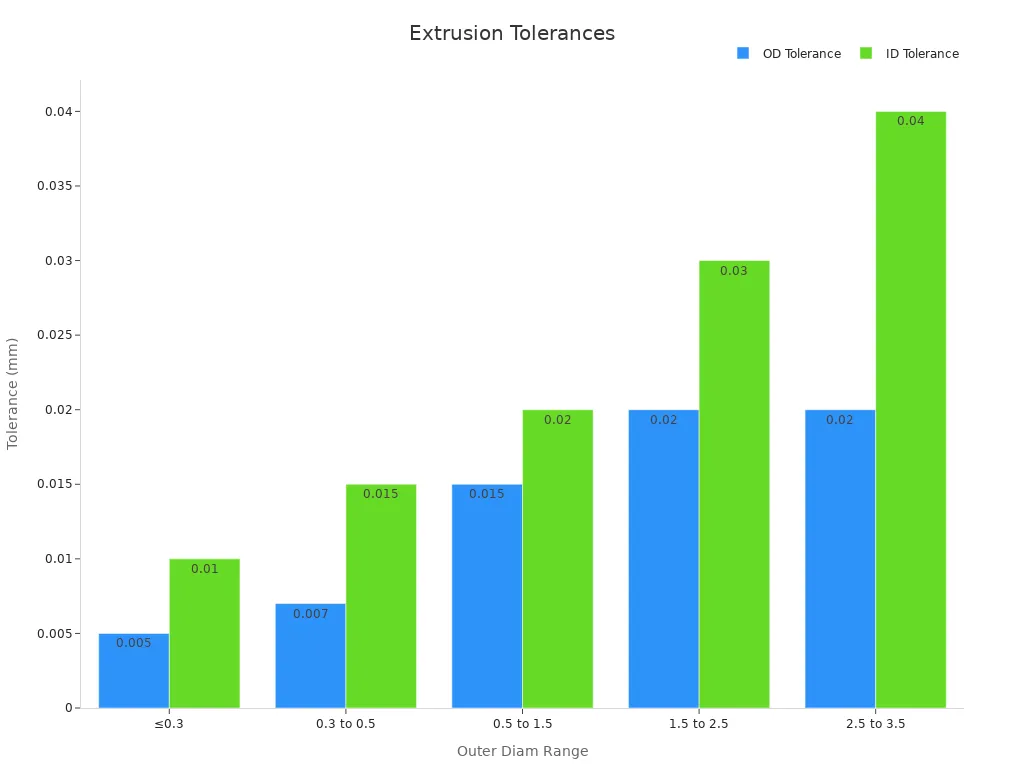
Manufacturers adhere to strict industrial benchmarks during the extrusion process. Air pressure, screw speed, and puller speed are carefully controlled to achieve consistent tube dimensions. Wall thickness tolerances do not exceed ±15 µm, and diameter tolerances remain within ±20 µm. Compliance with medical-grade standards such as ISO 13485 and FDA 21 CFR Part 820 ensures the safety and reliability of the tubing. Automated AI-driven inspection systems monitor tubing dimensions and surface quality in real time, reducing human error and enabling early defect detection.
Initial Tube Shaping
Initial tube shaping during extrusion determines the final performance of ultra-thin nitinol tubing. Engineers use microextrusion processes to form the first seamless tube, setting the stage for subsequent manufacturing steps. Precise control during this stage directly impacts the tubing’s fatigue life, mechanical strength, and superelasticity. Finite element analysis helps optimize extrusion parameters, reducing friction and deformation during tube formation.
Dimensional tolerances for both outer and inner diameters are tightly controlled, often within ±0.005 mm for small diameters. Real-time monitoring systems detect wall thickness variations as small as ±5 µm, allowing immediate adjustments to maintain uniformity. Quality control metrics such as tensile strength (500–900 MPa), local strains up to 6%, and cycles to failure up to 10⁷ confirm the tubing’s durability and performance.
Fatigue life improvements result from precise alloying and extrusion control.
Cold working and heat treatment after extrusion optimize superelasticity and shape memory.
Compliance with ASTM F2063 and ISO standards ensures chemical composition, mechanical properties, and biocompatibility meet stringent requirements.
Precision in initial tube shaping enables the tubing to meet the demands of neurovascular stents, including fatigue resistance, shape recovery, and durability.
Microextrusion and microextrusion processes allow the manufacture of thin walled tubes with exceptional consistency. The manufacturing process leverages advanced controls and real-time feedback to produce nitinol tubing that meets the strict requirements of microcatheter applications. Each step in the process, from billet preparation to initial tube shaping, contributes to the overall quality and reliability of ultra-thin nitinol tubing.
Drawing and Wall Thinning
Multi-Stage Drawing
Engineers rely on a multi-stage drawing process to refine nitinol tubing for microcatheter applications. This process involves pulling the tubing through a series of progressively smaller dies. Each stage reduces the diameter and wall thickness while maintaining the structural integrity of the nitinol. The multi-stage approach prevents excessive stress on the tubing, which could cause cracks or compromise superelasticity.
During manufacturing, technicians use cold working to enhance the microstructure of nitinol. This step increases fatigue life and improves the mechanical properties of the tubing. The process also limits porosity and non-metallic inclusions, which strengthens the tubing and supports long-term durability. Manufacturers monitor each stage with advanced measurement tools to ensure dimensional accuracy and mechanical stability.
Laser micrometers measure the outer diameter with high precision.
Optical comparators confirm dimensional conformity.
Bore gauges check the inner diameter.
Ultrasonic thickness gauges monitor wall thickness, maintaining tolerances as tight as ±0.0005" and wall thickness within 0.01 mm.
Strain recovery testing demonstrates a recovery ratio of 98% and strain recovery of 5.62%, confirming the superelastic performance of the tubing.
Surface roughness is controlled to Ra ≤ 0.1 μm, which enhances corrosion resistance and biocompatibility.
Non-destructive testing methods, such as ultrasonic and eddy current testing, verify structural integrity throughout the drawing stages.
Manufacturers follow ASTM and ISO standards, ensuring the nickel-titanium alloy composition remains consistent at 55-56% nickel by weight. Heat treatment after drawing stabilizes phase transformation behavior, resulting in load-bearing capacities exceeding 1400 N. These steps guarantee that superelastic nitinol tubing meets the demanding requirements of cardiovascular stents and microcatheters.
The multi-stage drawing process transforms nitinol into thin walled tubes with precise dimensions, exceptional mechanical stability, and reliable superelastic properties.
Achieving Uniform Thickness
Uniform wall thickness is critical for the performance and safety of nitinol tubing in microcatheter applications. Variations in wall thickness can lead to weak points, reducing the tubing’s ability to withstand repeated flexing and high pressures. Engineers optimize the drawing process parameters to achieve consistent wall thickness across the entire length of the tubing.
The following table compares wall thickness deviation under different process conditions:
Parameter Condition | Wall Thickness Deviation (%) |
|---|---|
Proper Parameters | 4.77 |
Improper Parameters | 12.03 |
This data shows that proper control during manufacturing significantly reduces wall thickness deviation. Tight tolerances ensure that thin walled tubes maintain their strength and flexibility, which is essential for navigating complex vascular pathways.
Manufacturers use real-time monitoring systems to detect even the smallest variations in wall thickness. These systems allow immediate adjustments, preventing defects and ensuring that every piece of tubing meets strict quality standards. Consistent wall thickness also improves the fatigue resistance and superelasticity of nitinol tubing, supporting its use in advanced medical devices.
Uniform wall thickness and tight tolerances are not just technical achievements—they are essential for patient safety and the long-term reliability of microcatheters.
Heat Treatment and Shape Setting
Annealing
Engineers use annealing as a critical step in the manufacturing process for thin-wall nitinol tubing. This heat treatment relieves residual stresses from cold working and refines the microstructure. By heating the tubing to precise temperatures, usually around 450°C, and then cooling it under controlled conditions, they enhance the superelasticity and shape-memory capabilities of the material. Differential scanning calorimetry (DSC) measurements show that annealing shifts the transformation temperatures of nitinol, which directly improves its mechanical performance. For example, annealing for one or two hours increases the austenite finish (AF) temperature from 23°C in untreated samples to over 41°C, indicating a more stable phase transformation.
Nitinol Condition | AF (°C) | AS (°C) | RF (°C) | RS (°C) |
|---|---|---|---|---|
Untreated | 23 | 16 | 9 | 19 |
Annealed 1 hour | 41 | 33 | 30 | 38 |
Annealed 2 hours | 43 | 38 | 33 | 40 |
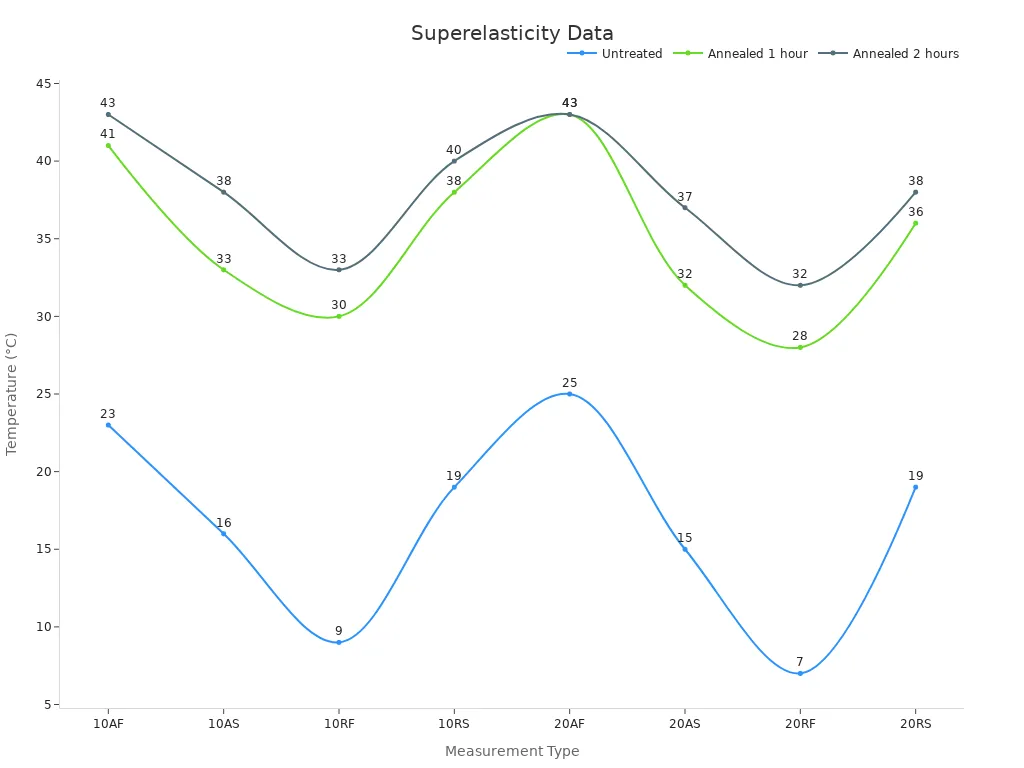
The process parameters, such as annealing temperature and cooling rate, play a vital role. Faster cooling, like water quenching, suppresses unwanted precipitates and increases microhardness, which strengthens the tubing. Slower cooling promotes coarser precipitates, affecting the tubing’s flexibility and kink resistance. Studies confirm that higher annealing temperatures and longer times increase radial force, which is essential for the mechanical performance of microcatheter tubing.
Shape Memory Properties
Heat treatment not only enhances superelasticity but also sets the desired shape of nitinol tubing. During shape setting, engineers form the tubing into precise geometries and then heat it to lock in those shapes. This process gives the tubing its unique shape-memory capabilities, allowing it to recover its original form after deformation. Quantitative research shows that controlling annealing parameters, such as temperature and cooling rate, directly influences the phase transformation behavior and the tubing’s ability to return to its preset shape.
After heat treatment, the microstructure of nitinol changes significantly. Grain size increases, and the distribution of precipitate phases becomes more uniform. These changes improve the stability of superelasticity and the tubing’s resistance to kinking. Mechanical testing demonstrates that heat-treated tubing achieves higher microhardness and better ductility, supporting its use in demanding medical environments. The applications of superelastic nitinol tubing rely on these enhanced properties to navigate complex vascular pathways and maintain performance over millions of cycles.
Proper heat treatment ensures that thin-wall nitinol tubing delivers reliable shape-memory capabilities, flexibility, and mechanical performance for advanced microcatheter applications.
Surface Finishing and Quality Control

Polishing and Cleaning
Surface finishing plays a vital role in preparing nitinol tubing for medical use. After extrusion, engineers focus on polishing to achieve a smooth, defect-free surface. This step reduces surface roughness by approximately 12-fold, reaching an average of 0.15 ± 0.06 µm. Such smoothness falls below the 0.2 µm threshold, which is critical for limiting microbial adhesion and plaque retention. Enhanced surface quality directly improves biocompatibility, making the tubing safer for implantation.
Cleaning follows polishing to remove any remaining contaminants. Technicians use a combination of chemical and mechanical cleaning protocols. The most effective method, combining chemical and mechanical cleaning, results in the lowest residual biomass and live/dead cell ratios. This approach ensures minimal microbial presence on the tubing surface, further supporting biocompatibility.
Cleaning Protocol | Residual Biomass (OD570) | Live/Dead Cell Ratio |
|---|---|---|
Immersion (ICP) | 1.77 ± 0.33 | 22.03 ± 7.45 |
Chemical (CCP) | 1.25 ± 0.28 | 1.81 ± 0.57 |
Mechanical (MCP) | 0.75 ± 0.23 | 7.20 ± 2.74 |
Chemical + MCP | 0.33 ± 0.16 | 0.42 ± 0.22 |
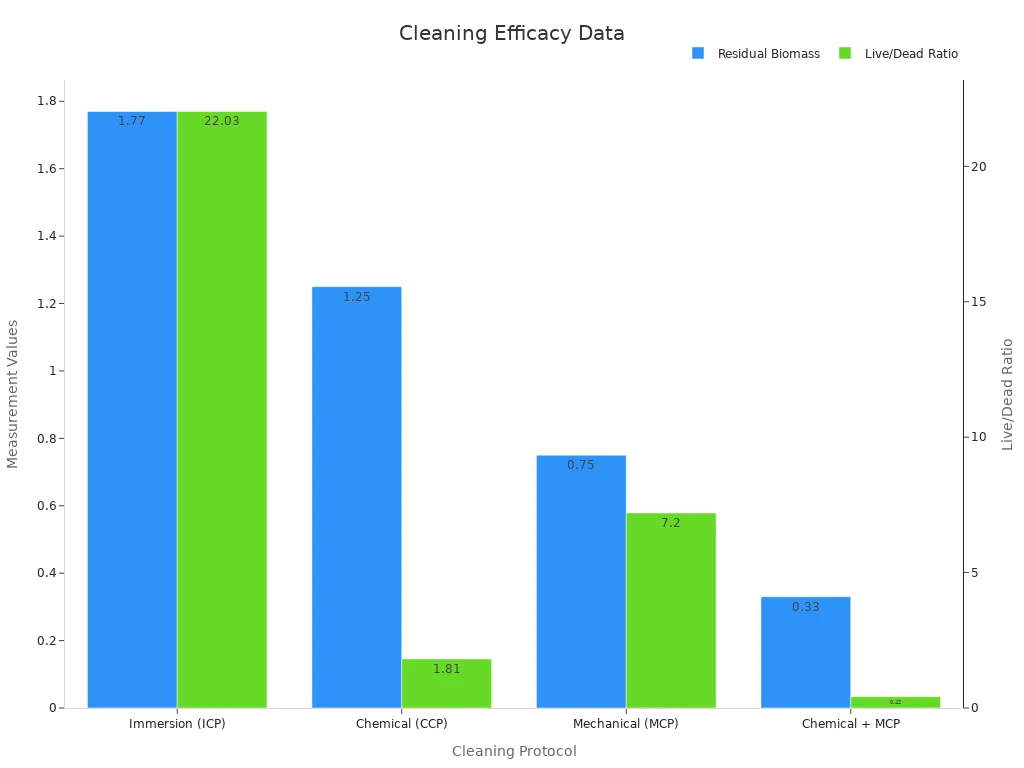
Engineers verify surface improvements using visual inspection, tactile assessment, surface profilometry, and microscopy. These methods confirm that the tubing meets strict standards for biocompatibility and smoothness.
Inspection and Testing
Quality control ensures that every piece of nitinol tubing meets the highest standards. After extrusion and surface finishing, technicians use several protocols to detect defects and confirm performance. Electropolishing removes microcracks and contaminants, which enhances both mechanical properties and biocompatibility. Chemical etching targets surface imperfections, improving fatigue resistance.
Non-destructive testing methods, such as ultrasonic and eddy current testing, identify internal and surface defects early. Visual inspections catch visible flaws, while advanced laser machining minimizes heat-affected zones and microcracks during cutting and welding. Technicians also use metallography and scanning electron microscopy to verify the removal of heat-affected zones.
Quality Control Protocol | Description and Statistical Support |
|---|---|
Electropolishing and Passivation | Removes microcracks, deburrs, and improves corrosion resistance and biocompatibility; considered best finishing method. |
100% Dimensional Inspection | Critical for verifying consistent, high-quality components meeting design specs; reduces patient risk. |
Validation and Inspection per ISO 13485:2012 | Ensures manufacturing processes and tooling are within tested control limits; requires experienced validation teams. |
Metallography and Scanning Electron Microscopy | Verifies removal of heat-affected zones (HAZ) to prevent microcracks. |
Corrosion Testing (ASTM F2129) | Statistically verifies corrosion resistance of finished nitinol tubing. |
Bend and Free Recovery Tests | Confirms material transformation temperatures and mechanical properties to ensure surface integrity. |
Comprehensive inspection and testing protocols guarantee that nitinol tubing produced through extrusion and advanced surface finishing meets the strict requirements for biocompatibility, durability, and safety in microcatheter applications.
Manufacturing thin-wall nitinol tubing for microcatheter applications involves precise extrusion, multi-stage drawing, and advanced heat treatment. Each step ensures tubing meets the strict demands of high-performance catheters and catheter manufacturing. Engineers use real-time monitoring and rigorous testing to achieve tight tolerances, high tensile strength, and excellent fatigue resistance. The table below summarizes key performance indicators that support the advantages in minimally invasive procedures and minimally invasive surgery:
Performance Indicator | Description |
|---|---|
Fatigue Life Ranking | Highest for custom tubing in all applications |
Tensile Strength | 500–900 MPa for high-performance catheters |
Superelasticity | Critical for flexibility in catheters and applications |
Biocompatibility | Safe for all medical applications |
Corrosion Resistance | Enhanced for long-term use in minimally invasive surgery |
Each stage in the manufacturing process guarantees that thin-wall nitinol tubing for microcatheter applications delivers reliability, safety, and high-performance in demanding medical applications.
FAQ
What makes thin-wall nitinol tubing ideal for microcatheter applications?
Thin-wall nitinol tubing offers superelasticity and biocompatibility. These properties make it suitable for demanding applications. The tubing can flex repeatedly without permanent deformation. Medical professionals rely on this tubing for advanced catheters in cardiovascular and neurovascular applications.
How do manufacturers ensure tubing meets strict tolerances for medical applications?
Manufacturers use advanced extrusion and drawing processes. Real-time monitoring systems check tubing dimensions. Automated inspection detects defects early. These steps guarantee tubing meets the tight tolerances required for catheters and other medical applications.
Why is uniform wall thickness important in tubing for microcatheter applications?
Uniform wall thickness prevents weak points in tubing. This consistency supports high fatigue resistance and flexibility. Reliable tubing performance is essential for catheters used in minimally invasive applications. Engineers monitor wall thickness throughout production to maintain quality.
What role does surface finishing play in tubing for medical applications?
Surface finishing removes imperfections from tubing. Polishing and cleaning improve biocompatibility. Smooth tubing surfaces reduce microbial adhesion. These enhancements make tubing safer for implantation in catheters and other medical applications.
How does heat treatment affect the properties of nitinol tubing in catheter applications?
Heat treatment refines the microstructure of tubing. This process enhances superelasticity and shape memory. Tubing can recover its original shape after deformation. These properties are critical for catheters and other applications that require flexibility and durability.
See Also
Comprehensive Process for Creating Nitinol Microtubing in Neurovascular Use
Understanding The Manufacturing Process Of Nitinol Tubing For Medicine
Detailed Guide To Selecting Appropriate Nitinol Tubing For Use
Complete Instructions For Producing Microcatheters Efficiently And Safely
The Importance Of Nitinol Tubing In Minimally Invasive Treatments

