How Superelastic Nitinol Tubing is Manufactured for Medical Devices

Superelastic Nitinol tubing for medical devices plays an essential role in the manufacturing process due to its remarkable properties. Nitinol, an alloy of nickel and titanium, exhibits superelasticity and shape memory, making it ideal for applications requiring flexibility and precision. The superelastic Nitinol tubing can return to its original shape after deformation, ensuring durability in demanding medical environments. Manufacturers use advanced techniques to create medical-grade tubing that meets strict standards for biocompatibility and performance. These processes ensure that superelastic Nitinol tubing for medical devices remains indispensable in the production of reliable and innovative medical devices.
Key Takeaways
Superelastic Nitinol tubing is made from a nickel-titanium mix. It is flexible and remembers its shape, perfect for medical tools.
The alloy must be very pure. Makers use special methods like vacuum arc remelting to remove dirt and make it safe for the body.
Cold working makes the tubing super stretchy. It can handle stress and go back to its shape after bending.
Electropolishing makes the tubing's surface smooth. This helps it work well in the body and lowers the chance of bad reactions.
Careful quality checks, like non-destructive testing, make sure the tubing is safe and meets medical standards.
Raw Material Selection in Nitinol Tubing Manufacturing
The production of superelastic nitinol tubing for medical devices begins with the careful selection of raw materials. This step is critical to ensure the final product meets the stringent requirements of medical device manufacturing. The unique properties of nitinol, such as superelasticity and shape memory, depend heavily on the precise composition and purity of the nickel-titanium alloy used.
Nickel-Titanium Composition and Its Role in Superelasticity
Nitinol, a shape memory alloy, derives its remarkable properties from its nickel-titanium composition. Typically, the alloy contains nearly equal parts of nickel and titanium, with slight variations tailored to specific applications. This precise balance is essential for achieving the superelastic behavior that allows nitinol tubing to return to its original shape after deformation. The nickel-titanium alloy's ability to undergo phase transformations between austenite and martensite phases under stress is what gives it both superelasticity and shape memory.
Manufacturers like AccuPath prioritize the consistency of this composition to ensure the tubing performs reliably in medical applications. Even minor deviations in the alloy's ratio can significantly impact its mechanical properties, making rigorous control during the initial stages of nitinol tubing manufacturing indispensable.
Ensuring Purity for Medical-Grade Nitinol Tubing
Purity plays a pivotal role in producing medical-grade nitinol tubing. Contaminants or impurities in the alloy can compromise its biocompatibility and mechanical performance, which are critical for medical applications. To achieve the required purity, manufacturers implement stringent quality control measures throughout the production process.
The vacuum arc remelting (VAR) process is a key step in maintaining purity. This method eliminates contaminants and ensures a homogenous alloy structure.
Oxygen and carbon levels are carefully controlled during melting to prevent the formation of hard inclusions, which could reduce the tubing's durability.
Ultrasonic testing is employed to detect inconsistencies within the material, while eddy current testing identifies surface irregularities. These non-destructive testing methods ensure the tubing's structural integrity and reliability.
Compliance with industry standards, such as those set by ASTM International and ISO, further guarantees the safety and effectiveness of medical-grade nitinol tubing. Manufacturers like AccuPath adhere to these benchmarks to deliver tubing that meets the highest quality standards. Their commitment to precision and purity makes them a trusted partner in the medical device manufacturing industry.
By focusing on the composition and purity of the nickel-titanium alloy, manufacturers lay the foundation for producing superelastic nitinol tubing that meets the rigorous demands of medical applications. This meticulous attention to detail ensures the tubing's reliability, biocompatibility, and performance in life-saving medical devices.
Melting and Shaping of Superelastic Nitinol Tubing

Vacuum Melting and Ingot Formation
The manufacturing process of nitinol tubing begins with vacuum melting. This step ensures the alloy achieves the required purity and homogeneity. Manufacturers use a vacuum induction melting (VIM) process to combine nickel and titanium in precise proportions. The controlled environment prevents contamination from oxygen or other impurities.
Once melted, the alloy is poured into molds to form ingots. These ingots serve as the foundation for creating superelastic nitinol tubing for medical devices. The ingot formation process is critical because it determines the alloy's structural integrity. Any inconsistencies at this stage could affect the tubing's performance in medical applications.
After the ingots cool, they undergo inspection to ensure they meet strict quality standards. Manufacturers often use ultrasonic testing to detect internal flaws. This step guarantees that the nitinol maintains its superelastic and shape memory properties throughout the production process.
Extrusion and Gun-Drilling for Initial Tube Shapes
After forming the ingots, manufacturers shape them into tubes through extrusion. This process involves heating the ingots and forcing them through a die to create a rough tube shape. Extrusion reduces the ingot's diameter while maintaining its structural properties.
For medical-grade nitinol tubing, precision is essential. Gun-drilling refines the tube's internal diameter by removing excess material. This technique creates a smooth and uniform inner surface, which is crucial for medical applications.
The combination of extrusion and gun-drilling ensures the tubing achieves the desired dimensions and mechanical properties. These steps lay the groundwork for producing high-quality superelastic nitinol tubing that meets the rigorous demands of the medical industry.
Heat Treatment and Shaping Processes
Cold Working to Enhance Superelastic Properties
Cold working plays a vital role in enhancing the superelastic properties of nitinol tubing. This process involves deforming the material at room temperature to improve its mechanical characteristics. Manufacturers apply controlled pressure to the nitinol tubing, which strengthens the alloy by introducing dislocations into its crystal structure. These dislocations increase the tubing's ability to withstand stress and improve its superelasticity.
During cold working, the tubing undergoes processes such as rolling, drawing, or swaging. Each method reduces the tubing's diameter while maintaining its structural integrity. The repeated deformation refines the grain structure of the nitinol, which is essential for achieving the desired superelastic behavior. This step ensures that the tubing can return to its original shape after being bent or stretched, making it ideal for medical applications.
Cold working also allows manufacturers to tailor the mechanical properties of the tubing to specific requirements. By adjusting the amount of deformation, they can control the stiffness, flexibility, and strength of the nitinol tubing. This customization is crucial for creating medical devices that require precise performance, such as stents or guidewires.
Annealing and Tube Drawing for Precision
Annealing is a heat treatment process that follows cold working. It involves heating the nitinol tubing to a specific temperature and then cooling it at a controlled rate. This step relieves the internal stresses introduced during cold working and restores the material's ductility. Annealing also enhances the tubing's shape memory properties, ensuring it can return to its original form after deformation.
Manufacturers carefully monitor the annealing process to achieve the desired balance between flexibility and strength. The temperature and duration of annealing are critical factors that influence the final properties of the nitinol tubing. For medical applications, precision is paramount, and even slight variations in the annealing process can impact the tubing's performance.
After annealing, the tubing undergoes tube drawing to achieve the required dimensions and surface finish. Tube drawing involves pulling the tubing through a die to reduce its diameter and improve its uniformity. This process ensures that the tubing meets the tight tolerances required for medical devices. The combination of annealing and tube drawing results in nitinol tubing with exceptional precision and reliability.
Manufacturers use advanced equipment and techniques to perform these processes with high accuracy. The resulting superelastic nitinol tubing for medical devices exhibits the necessary properties for demanding applications. Its ability to maintain performance under stress and its biocompatibility make it a preferred choice in the medical industry.
Surface Treatment for Medical-Grade Nitinol Tubing
Electropolishing for Biocompatibility
Electropolishing enhances the biocompatibility of nitinol tubing by refining its surface. This process involves using an electrochemical method to remove microscopic imperfections, resulting in a smooth and uniform finish. The smoother surface reduces the risk of adverse reactions when the tubing is implanted in the human body. Polished nitinol surfaces integrate better with surrounding tissues, improving their compatibility with biological systems.
Surface characteristics play a vital role in medical applications. Titanium oxides formed during electropolishing enhance the tubing's interaction with biological environments. These oxides create a protective layer that minimizes harmful ion release, ensuring the tubing remains safe for long-term use. Studies have shown that electropolished nitinol tubing exhibits superior performance in medical devices, such as stents and catheters, due to its improved biocompatibility.
Manufacturers prioritize electropolishing to meet stringent medical standards. This step ensures the tubing performs reliably in critical applications, where safety and compatibility are paramount.
Passivation to Prevent Corrosion
Passivation protects nitinol tubing from corrosion by creating a chemically stable surface. This process involves treating the tubing with an acid solution to remove contaminants and enhance its resistance to oxidation. The result is a thin, protective layer of titanium oxides that shields the tubing from environmental factors.
Corrosion resistance is essential for medical-grade nitinol tubing. Without proper treatment, the tubing could release nickel ions, which may cause adverse reactions in patients. Passivation prevents this by stabilizing the surface and reducing the risk of ion release. This makes the tubing safer for use in medical devices, especially those implanted for extended periods.
Passivation also improves the durability of nitinol tubing. The protective layer ensures the tubing maintains its structural integrity under stress, making it suitable for demanding medical applications. Manufacturers incorporate passivation into the production process to meet industry standards and deliver tubing that performs reliably in life-saving devices.
Quality Control in Nitinol Tubing Manufacturing
Non-Destructive Testing for Structural Integrity
Non-destructive testing plays a critical role in ensuring the structural integrity of nitinol tubing. These methods allow manufacturers to identify internal defects without damaging the material. Ultrasonic inspection is one of the most effective techniques used in nitinol tubing manufacturing. It detects inconsistencies within the tubing, such as voids or cracks, that could compromise its performance.
Quality control measures ensure the reliability of nitinol tubing by thoroughly testing each batch for mechanical properties, dimensional accuracy, and surface quality. Manufacturers prioritize these tests to maintain consistency in performance.
Key non-destructive testing methods include:
Ultrasonic testing for internal flaws.
X-ray and sound wave tests for material checks.
Strength tests to evaluate stretching and holding capabilities.
These rigorous quality control measures ensure that nitinol tubing meets the high standards required in the medical device industry.
Dimensional Inspections to Ensure Precision
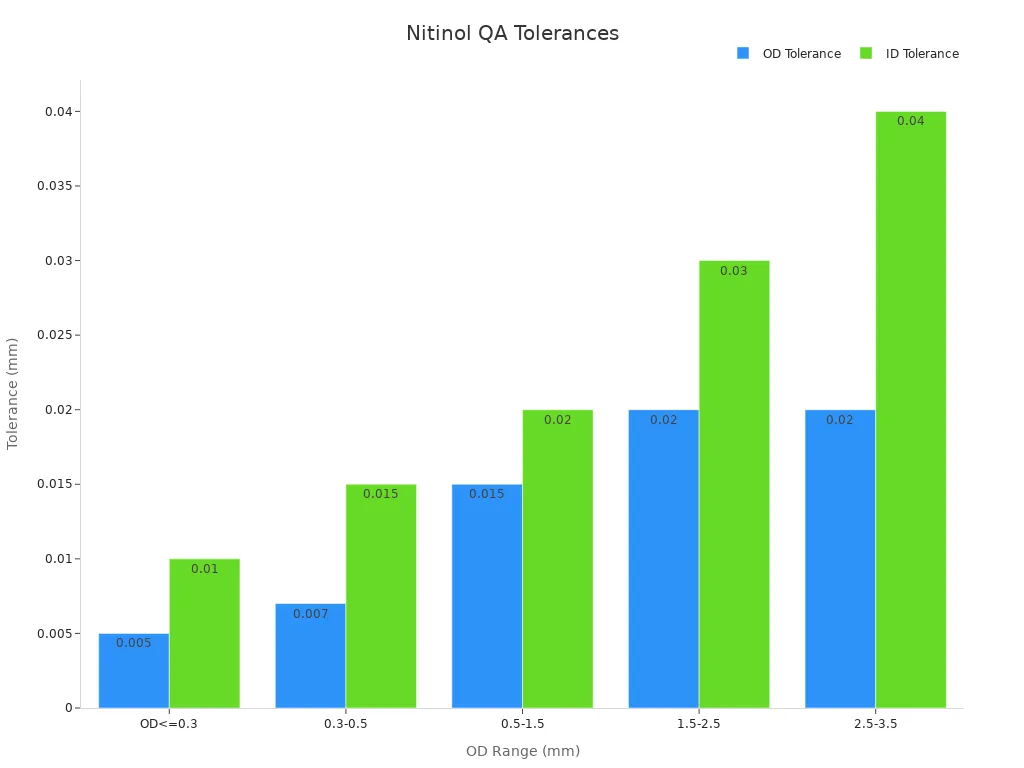
Dimensional inspections are essential for achieving the precise tolerances required in medical device manufacturing. Nitinol tubing must meet strict specifications for outer diameter (OD) and inner diameter (ID) to ensure compatibility with medical devices. Manufacturers use advanced tools to measure these dimensions accurately.
The table below highlights the tolerances maintained during nitinol tubing manufacturing:
Outer Diameter (OD) Range (mm) | OD Tolerance (mm) | ID Tolerance (mm) |
|---|---|---|
OD≤0.3 | ±0.005 | ±0.010 |
0.3≤OD≤0.5 | ±0.007 | ±0.015 |
0.5≤OD≤1.5 | ±0.015 | ±0.020 |
1.5≤OD≤2.5 | ±0.020 | ±0.030 |
2.5≤OD≤3.5 | ±0.020 | ±0.040 |
These precise measurements ensure that nitinol tubing performs reliably in medical applications. Manufacturers also conduct ultrasonic testing to verify wall thickness and ensure uniformity.
Compliance with Medical Industry Standards
Compliance with industry standards is a cornerstone of nitinol tubing manufacturing. Organizations like ASTM International and ISO establish guidelines for material quality, biocompatibility, and performance. Manufacturers adhere to these standards to ensure their tubing meets the stringent requirements of the medical device industry.
Proof of quality is evident in the materials used and the processes followed. For example:
Proof of Quality | What It Ensures |
|---|---|
Cleaner materials | Longer-lasting tubes that meet ASTM standards |
By following these standards, manufacturers guarantee that nitinol tubing is safe, durable, and effective for use in life-saving medical devices.
The manufacturing of superelastic nitinol tubing for medical devices involves a series of precise steps. From selecting high-purity nitinol to applying advanced surface treatments, each process ensures the tubing meets the rigorous demands of medical applications. Manufacturers prioritize quality control to guarantee reliability and safety. These efforts result in tubing that performs consistently under stress and integrates seamlessly into medical devices. Superelastic nitinol tubing remains indispensable in the medical field due to its unique properties and exceptional performance.
FAQ
What makes Nitinol tubing superelastic?
Nitinol tubing achieves superelasticity through its unique nickel-titanium alloy composition. This material undergoes phase transformations between austenite and martensite under stress, allowing it to return to its original shape after deformation. Manufacturers enhance this property through precise heat treatments and cold working processes.
Why is purity important in medical-grade Nitinol tubing?
Purity ensures the biocompatibility and mechanical reliability of Nitinol tubing. Impurities can compromise its performance and safety in medical applications. Manufacturers use processes like vacuum arc remelting (VAR) to eliminate contaminants and meet stringent medical industry standards.
How does electropolishing improve Nitinol tubing?
Electropolishing removes surface imperfections, creating a smooth and uniform finish. This process enhances biocompatibility by reducing the risk of adverse reactions in the body. It also forms a protective titanium oxide layer, which minimizes harmful ion release and improves corrosion resistance.
What quality control methods are used in Nitinol tubing production?
Manufacturers use non-destructive testing methods like ultrasonic inspection and dimensional checks. These tests identify internal flaws, ensure precise dimensions, and verify structural integrity. Adhering to ASTM and ISO standards guarantees the tubing meets medical industry requirements.
Can Nitinol tubing be customized for specific medical devices?
Yes, manufacturers can tailor Nitinol tubing to meet specific requirements. Adjustments in cold working, annealing, and tube drawing processes allow customization of properties like flexibility, strength, and dimensions. This ensures compatibility with various medical devices, such as stents and guidewires.

