Beginner’s Guide to Superelastic Nitinol Tubing in Stroke Intervention

Superelastic nitinol tubing plays a critical role in the development of advanced medical devices, especially in Superelastic Nitinol Tubing for Stroke Intervention Stents. Nitinol provides unique properties such as shape memory and superelasticity, which allow stents to adapt within arteries and support effective stroke treatment. Clinical studies and imaging research confirm nitinol’s ability to promote vascular healing and safe device performance.
Precision manufacturing ensures nitinol tubing meets strict medical standards.
Evidence Aspect
Description
Clinical Reliability
Implantation in 30 sheep with iliac vein stents showed no damage or fractures immediately post-implantation.
Manufacturing Quality
Precision engineering and advanced surface finishing increase durability and reduce corrosion susceptibility.
Material Properties
Fatigue strain limits between 0.4–0.8% and excellent corrosion resistance support reliable medical devices.
Nitinol tubing outperforms traditional metals in flexibility, durability, and biocompatibility, making it essential for modern medical stents.
Key Takeaways
Superelastic nitinol tubing offers unmatched flexibility and shape memory, allowing stents to adapt and recover inside blood vessels during stroke treatment.
Nitinol tubing provides superior durability, corrosion resistance, and biocompatibility, making it safe and reliable for long-term medical implants.
Advanced manufacturing processes like vacuum melting, gun drilling, laser cutting, and heat treatment ensure precise, high-quality nitinol tubing for medical devices.
Strict quality control and compliance with medical standards guarantee that nitinol tubing meets safety and performance requirements for stroke intervention stents.
Choosing nitinol tubing from trusted suppliers with proven quality systems supports better patient outcomes and advances stroke intervention technology.
What Is Nitinol Tubing?
Nitinol Basics
Nitinol tubing stands at the forefront of medical device innovation. This tube, crafted from a nearly equiatomic nickel-titanium alloy, forms the backbone of many minimally invasive procedures. Nitinol tubing for precision neurosurgery relies on its unique combination of mechanical and physical properties. The material’s shape memory and superelasticity distinguish it from conventional metals. Nitinol tubing for precision neurosurgery demonstrates high fatigue resistance, superior corrosion resistance, and remarkable biocompatibility. These features make nitinol tubing the preferred choice for stents, filters, and other implantable devices.
Nitinol tubing for precision neurosurgery offers a high strength-to-weight ratio, electrical conductivity, and radiopacity, which ensures visibility under X-ray imaging.
The following table summarizes key technical specifications and manufacturing processes for nitinol tubing used in stroke intervention devices:
Specification/Process | Details |
|---|---|
Elastic Modulus | 28 GPa |
Poisson's Ratio | 0.3 |
Material Density | 6.45 g/cc |
Tensile Strength | 100 MPa |
Stent Strut Dimensions | Thickness: 0.08 mm, Width: 0.075 mm, Length: 0.15 mm |
Laser Machining Method | Photolithography-based laser processing (55 W, 16.5 µm, oxygen cooling) |
Chemical Treatment | Cleaning and etching to remove oxides and residual stress |
Heat Treatment | High-vacuum annealing at 510 °C for 10 minutes |
Nitinol tubing for precision neurosurgery undergoes rigorous ultrasonic inspection and functional testing to ensure mechanical integrity and uniform wall thickness. The technology behind nitinol tubing ensures that each tube meets the demands of advanced medical applications.
Superelasticity Explained
Superelastic nitinol exhibits a remarkable ability to recover from significant deformation. This property arises from a reversible phase transformation between austenite and martensite structures within the alloy. When nitinol tubing for precision neurosurgery experiences mechanical stress, it transforms its internal structure, allowing the tube to bend and flex without permanent damage. This superelastic behavior enables nitinol tubing to conform to complex vascular pathways during stroke intervention.
Nitinol shape memory alloys provide constant unloading stresses over large strains, which is essential for stents that must expand and adapt to vessel walls. The technology used in manufacturing nitinol tubing, including precise alloy composition and heat treatment, enhances superelasticity and durability. Nitinol tubing maintains its performance through repeated cycles of deformation and recovery, making it ideal for devices that require flexibility and resilience.
Nitinol tubing’s superelasticity supports long-term reliability in the human body.
The combination of corrosion resistance, fatigue strength, and biocompatibility ensures safe and effective use in medical devices.
Nitinol tubing, as a product of advanced nitinol shape memory alloys, continues to set the standard for innovation in stroke intervention and other biomedical fields.
Superelastic Nitinol Tubing for Stroke Intervention Stents

Why Use Nitinol in Stents
Superelastic nitinol tubing for stroke intervention stents has transformed the design and function of modern vascular implants. Nitinol tubing stands out due to its unique superelasticity and shape memory, which allow stents to adapt to the dynamic environment of blood vessels. Nitinol stent manufacturing leverages these properties to create devices that can be crimped into small diameters for delivery and then expand precisely at the target site. This process ensures that stents maintain vessel patency and resist deformation from physiological forces.
Nitinol tubing offers several advantages over conventional materials. The following table highlights key differences:
Property/Aspect | Superelastic Nitinol Tubing | Conventional Materials (e.g., Cobalt-Chrome) |
|---|---|---|
Elastic Strain | Exceeds 8% due to phase transformation | Approximately 1% |
Elastic Modulus | Non-linear, phase transformation driven | 200-240 GPa |
Wire Flexion | High, enabling crimping and self-expansion | Limited, prone to permanent deformation |
Stent Design Flexibility | Enables complex closed loop designs | Limited to open loop configurations |
Radial Force and Conformability | Enhanced due to superelasticity | Lower due to material stiffness |
Biocompatibility | Superior | Good but less optimal |
Durability, Corrosion, Fatigue | High, with optimized surface treatments | Variable, generally lower fatigue resistance |
MRI Compatibility | Compatible | Variable |
Nitinol stent manufacturing enables the production of stents with complex geometries, supporting advanced applications of nitinol tubing in medical devices. The technology behind nitinol tubing ensures high biocompatibility and durability, making it the preferred choice for stents and vascular implants.
Benefits in Stroke Intervention
Superelastic nitinol tubing for stroke intervention stents provides significant clinical and procedural benefits. Nitinol tubing allows stents to navigate tortuous vascular anatomy and maintain their structure under stress. Medical professionals choose nitinol for its ability to self-expand and conform to vessel walls, reducing the risk of restenosis and embolization.
Nitinol stents have lowered restenosis rates compared to balloon angioplasty alone in femoropopliteal arterial disease.
The superelasticity of nitinol tubing helps stents withstand flexion, compression, and torsion in challenging vascular segments.
Nitinol stent manufacturing reduces fracture risk, a common issue with less flexible materials.
Clinical guidelines recommend nitinol stents when balloon angioplasty results are unsatisfactory, supporting long-term vessel patency.
Nitinol tubing also supports advanced stent designs, such as double-layer micromesh configurations, which improve clinical outcomes in stroke intervention. Clinical trials, including the SAPPHIRE study, show that nitinol stents achieve similar stroke rates to surgical procedures over three years, with no significant difference in major adverse events. These results confirm the safety and efficacy of superelastic nitinol tubing for stroke intervention stents.
Note: Self-expanding nitinol stents enable easy delivery through microcatheters, even in complex cerebrovascular anatomy, and adapt to vessel movement for optimal performance.
Nitinol tubing, through precise manufacturing and production processes, continues to set the standard for medical devices in vascular applications. The combination of superelasticity, biocompatibility, and advanced technology ensures that stents and vascular implants deliver reliable results in stroke intervention.
Key Properties of Nitinol
Shape Memory
Nitinol stands out among medical materials due to its remarkable shape memory effect. This property allows nitinol tubing to return to its original shape after deformation when exposed to specific temperatures. Medical devices such as stents rely on this effect to expand inside the body and restore blood flow. Researchers have shown that nitinol undergoes phase transformations between martensite and austenite, triggered by temperature and mechanical load. These transformations influence the alloy’s hardness and Young’s modulus, making nitinol adaptable for various medical applications. The ability to control transformation temperatures by adjusting nickel content ensures that each shape memory alloy device performs reliably in clinical settings.
Shape memory enables nitinol stents to expand precisely at the target site.
Superelasticity allows nitinol to bend and flex without permanent damage, supporting devices like guidewires and catheters.
Biocompatibility
Biocompatibility remains a critical factor for all medical devices. Nitinol demonstrates excellent biocompatibility, making it suitable for long-term implantation in the human body. Surface treatments such as electropolishing and chemical passivation create a smooth, oxide-rich layer on nitinol tubing. This layer reduces nickel ion release and minimizes platelet adhesion, which lowers the risk of thrombosis. Manufacturing processes that include precise alloying and heat treatment further enhance nitinol’s safety profile. Clinical and animal studies confirm that nitinol stents cause less vessel wall injury and lower restenosis rates compared to other materials. Medical professionals trust nitinol for its proven record of safe interaction with human tissues.
Tip: Advanced surface treatments improve both biocompatibility and corrosion resistance, making nitinol ideal for cardiovascular and neurovascular devices.
Corrosion Resistance
Corrosion resistance is essential for any material used in medical environments. Nitinol’s unique composition of nickel and titanium provides outstanding resistance to corrosion, even in the challenging conditions of the human body. Electropolishing creates a thin, uniform oxide layer that protects the alloy from pitting and reduces nickel ion dissolution. In vitro studies show that nitinol tubing maintains its integrity under physiological conditions, outperforming stainless steel and other metals. The high breakdown potential of nitinol, as shown in comparative studies, highlights its durability in corrosive biological environments. Medical devices made from nitinol benefit from this resilience, ensuring long-term performance and patient safety.
Material | Breakdown Potential (V) |
|---|---|
304 Stainless Steel | 0.2 |
316 Stainless Steel | 0.4–0.48 |
Titanium | 9 |
Nitinol Alloy | 25 |
Nitinol’s biocompatibility and corrosion resistance make it a preferred choice for medical devices, especially those used in stroke intervention and other vascular procedures.
Manufacturing Process of Nitinol Tubing

The manufacturing process of nitinol tubing for stents involves a series of highly controlled steps. Each stage requires specialized equipment and strict process management to ensure the final product meets the demanding standards of medical applications. The following sections describe the core steps in nitinol stent manufacturing, highlighting the importance of precision, quality, and advanced fabrication techniques.
Alloying and Vacuum Melting
Manufacturers begin by selecting high-purity nickel and titanium. They carefully control the alloy composition to achieve the desired balance of flexibility, shape memory, and superelasticity. Vacuum melting, such as Vacuum Induction Melting (VIM), melts these metals in a vacuum environment. This process prevents contamination and removes impurities, resulting in a homogeneous alloy. Operators maintain precise temperatures, often around 800 °C, and control pressure to 3.25 MPa. This step ensures the nitinol alloy forms a solid structure with minimal flaws, which is essential for subsequent tubing fabrication and medical device performance. Dimensional control during vacuum melting eliminates contaminants and balances the nickel-titanium ratio, supporting optimal mechanical properties.
Gun Drilling
Gun drilling technology creates the initial hollow structure in nitinol bars. This specialized machining process produces precision holes, which serve as the starting point for tubing fabrication. Gun drilling allows for tight control of wall thickness and diameter, both critical for medical device specifications. After gun drilling, thermomechanical processing further enhances the shape memory and superelastic properties of the nitinol tubing. Bringing gun drilling in-house improves material availability, reduces lead times, and supports rapid prototyping. Automated long-stroke gun drilling increases manufacturing efficiency and meets the stringent demands of nitinol stent manufacturing.
Gun drilling enables the production of high-performance nitinol tubing by creating precise, hollow tubes from solid bars.
The process supports compliance with ASTM F2063 standards for medical-grade nickel titanium alloys.
Drawing and Sizing
After gun drilling, the tubing undergoes drawing and sizing. This process reduces the tube diameter and wall thickness to meet exact specifications. Manufacturers use a series of dies to draw the tubing, achieving tolerances as tight as ±0.005 mm. Shape-setting methods, either thermal or mechanical, fix the tubing’s final geometry. Surface quality and cleanliness are assessed through quantitative tests, including skin sensitization assays and microbiological cleanliness checks. Biocompatibility testing follows ISO 10993 standards, ensuring the tubing is safe for implantation. Mechanical and fatigue testing confirm the strength and durability of the nitinol tubing, while chemical analysis detects harmful extractables. Wear and debris analysis using microscopy prevents adverse tissue reactions. Traceability is maintained through durable laser marking, supporting regulatory compliance and quality control in nitinol stent production.
Laser Cutting for Stents
Laser cutting represents a critical step in nitinol stent manufacturing. High-powered lasers cut intricate stent designs into the nitinol tubing with exceptional precision. The process achieves accuracy levels of 10–20 microns and tolerances as tight as ±0.1 mm, ensuring a perfect fit and minimal material waste. Fiber laser cutting produces narrow kerf widths and avoids heat-affected zones, preserving the mechanical properties of the tubing. Statistical analyses confirm that laser cutting speed has a greater impact on surface quality than laser power. The process capability of fiber laser cutting meets the stringent standards required for cardiovascular stents.
Outer Diameter Range (mm) | Outer Diameter Tolerance (mm) | Inner Diameter Tolerance (mm) |
|---|---|---|
Up to 0.3 | ±0.005 | ±0.010 |
0.3 to 0.5 | ±0.007 | ±0.015 |
0.5 to 1.5 | ±0.015 | ±0.020 |
1.5 to 2.5 | ±0.020 | ±0.030 |
2.5 to 3.5 | ±0.020 | ±0.040 |
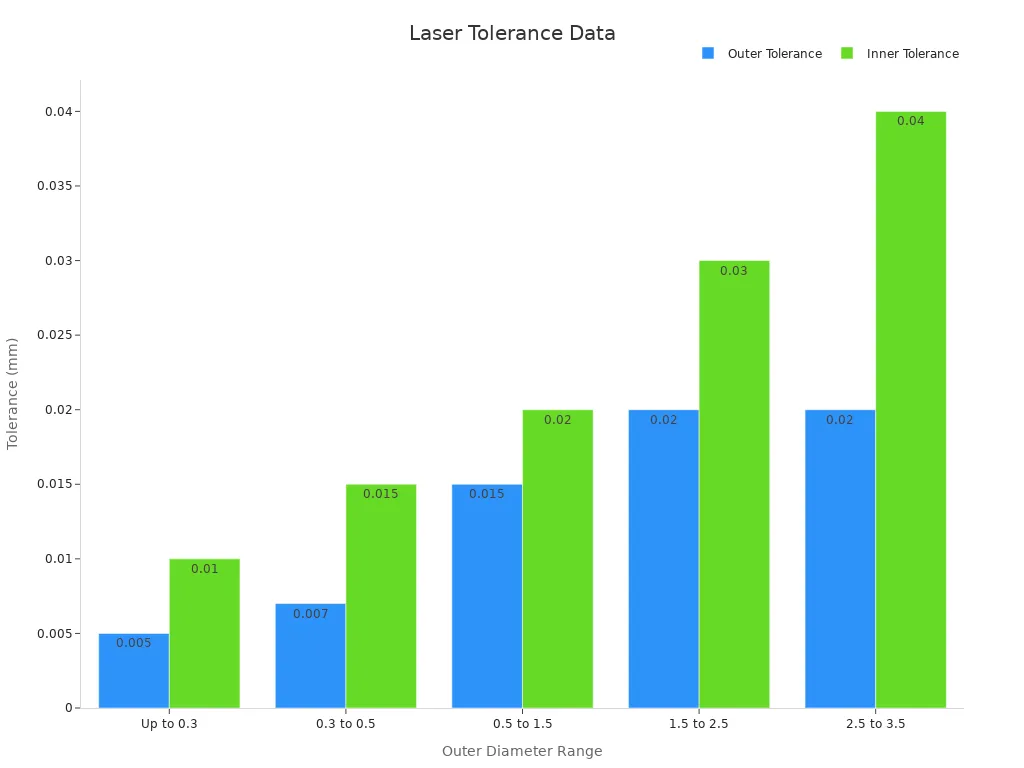
Laser cutting enables the fabrication of complex stent geometries, supporting advanced nitinol stent manufacturing and ensuring consistent quality.
Heat Treatment and Shape Setting
Heat treatment and shape setting define the final mechanical properties of nitinol tubing. Manufacturers heat the tubing at temperatures between 350–600 °C for controlled periods. This process creates Ti3Ni4 particles, modifies nickel content, and raises the martensite start (Ms) temperature. The result is enhanced shape memory and mechanical strength. Multi-step heat treatments can increase transformation temperatures and alter mechanical properties, making detailed thermal and mechanical characterization essential. The stress relaxation process during shape setting permanently fixes the desired stent shape. Manufacturers use custom tooling and precise temperature control to ensure consistent outcomes across production batches.
Parameter Studied | Description | Key Findings |
|---|---|---|
Chemical Composition | Ni50.8Ti vs. Ni50.6Ti alloys | Mechanical properties shift despite similar transformation temperatures |
Material Format | Wire vs. Tube | Tubing from different vendors shows significant variability |
Heat Treatment Time & Temp | 350–550 °C for wires; 250–600 °C for tubes | Strong influence on phase transformation and mechanical properties |
Vendor Variability | Tubing from two vendors with same specs | ~35 °C difference in Af temperature; different transformation pathways |
Multi-step vs. Single-step | Single vs. multi-step shape setting | Multi-step treatments lead to higher Af temperatures and altered properties |
Surface Finishing
Surface finishing improves the biocompatibility and corrosion resistance of nitinol tubing. The process includes rough and fine polishing, buffing, and electropolishing. Electropolishing removes surface imperfections and reduces nickel release, which enhances safety for implantation. Cleaning steps eliminate residual particles, ensuring surface purity. Dimensional accuracy is verified using laser micrometers and coordinate measuring machines. Mechanical properties are tested through tensile and fatigue tests, while shape memory and superelasticity are confirmed via Bend Free Recovery tests. Non-destructive testing methods such as X-rays and ultrasounds detect hidden defects. Compliance with ASTM F2063 and ISO 10993 standards ensures the tubing meets medical device requirements.
Measurement / Specification | Description / Purpose | Example Values / Notes |
|---|---|---|
Surface Roughness Reduction | Verifies improvement in surface finish after electropolishing | Roughness reduced from 80 Ra to 40 Ra |
Dimensional Accuracy | Ensures tubing meets tight tolerances for medical use | Laser micrometry for diameter, wall thickness checks |
Non-Destructive Testing (NDT) | Detects internal flaws and surface defects without damage | Ultrasonic testing, X-rays, ultrasounds |
Mechanical Property Testing | Confirms strength, elasticity, and fatigue resistance | Tensile tests, fatigue tests, Bend Free Recovery (BFR) |
Heat Treatment Parameters | Controls superelasticity and shape-setting | Shape-setting at 500°C for 30 minutes |
Compliance Standards | Ensures biocompatibility and safety | ISO 10993, ASTM F2063 |
Corrosion Resistance Evaluation | Confirms surface finishing effectiveness | Enhanced corrosion resistance after electropolishing |
Biocompatibility Testing | Validates safety for medical implantation | Tests per ISO 10993 standards |
Quality Control
Quality control in nitinol stent production ensures every nitinol tubing implant meets strict medical standards. Manufacturers perform 100% dimensional inspection on each component. They validate and inspect all manufacturing process steps and tooling within established control limits. Compliance with ISO 13485:2012 standards guides validation activities. In-house verification technologies, including metallography and scanning electron microscopy, confirm the removal of heat-affected zones. Corrosion testing follows ASTM F2129 protocols, while thermal transformation temperatures are measured using Bend Free Recovery and Differential Scanning Calorimetry. Electropolishing enhances corrosion resistance and biocompatibility by polishing, passivating, and removing micro-cracks. Manufacturers develop custom shape-setting tooling and qualify multiple raw material suppliers to maintain supply continuity. Rapid prototyping and iterative design verification support innovation in nitinol stent manufacturing.
Tip: Complete in-house manufacturing capability allows for full process control, ensuring consistent quality and reducing the risk of defects in medical grade nitinol tubing.
The manufacturing process of nitinol tubing for stents requires precise control, advanced fabrication techniques, and rigorous quality management. Each step, from alloying to final inspection, plays a vital role in producing safe, reliable, and high-performance nitinol stents for stroke intervention.
Choosing Nitinol Tubing for Stents
Key Specifications
Selecting nitinol tubing for stents requires careful attention to several technical and performance criteria. Manufacturers focus on properties that ensure safety, durability, and optimal function in medical applications. The following table summarizes the most critical specifications for nitinol tubing used in stents:
Specification Aspect | Key Details |
|---|---|
Fatigue Life | Custom tubing offers superior fatigue resistance, vital for high-performance stents. |
Tensile Strength | Ranges from 500 MPa to 900 MPa, supporting mechanical stress in medical devices. |
Shape Memory & Superelasticity | Essential for stents to recover shape and endure deformation. |
Material Purity | High purity reduces fracture risk and enhances durability. |
Surface Treatments | Electropolishing and coatings improve corrosion resistance and longevity. |
Compliance Standards | Adherence to ASTM F2063 and regulatory guidelines ensures safety. |
Customization | Tubing dimensions and finishes tailored for specific medical devices. |
Durability Testing | Accelerated fatigue and corrosion tests validate long-term performance. |
Manufacturers also consider the presence of inclusions or voids in nitinol tubing, as these can increase fracture risk under physiological stress. High purity nitinol enables tubing to withstand millions of deformation cycles, which is essential for stents, guidewires and catheters. Compliance with standards such as ASTM F2063 ensures that nitinol tubing meets strict requirements for chemical composition, mechanical properties, and biocompatibility. Accelerated durability testing, including fatigue and corrosion resistance assessments, confirms that nitinol tubing can endure the demands of medical environments.
Tip: Always verify that nitinol tubing has passed both mechanical and surface quality tests before use in stents or other medical devices.
Supplier Evaluation
Choosing the right supplier for nitinol tubing impacts the quality and reliability of stents and other medical devices. Leading suppliers demonstrate robust quality management and regulatory compliance. Key evaluation criteria include:
Quality Management System (QMS) aligned with industry standards.
Good Manufacturing Practices (GMP) to ensure cleanliness and process control.
Regulatory compliance with FDA and international guidelines.
Consistent process control and validation for specification adherence.
Audit and inspection history, including corrective actions.
Effective Corrective and Preventive Actions (CAPA) for quality issues.
Reliable delivery schedules and low defect rates.
Strong inventory management to prevent shortages.
Responsive customer service and technical support.
Post-sale support, including maintenance and complaint resolution.
Customization capabilities for tubing dimensions and finishes.
Investment in innovation and research for advanced nitinol tubing.
Positive reputation and client testimonials reflecting reliability.
Suppliers must deliver nitinol tubing with consistent superelasticity, shape memory, and corrosion resistance. These properties are essential for stents, guidewires and catheters, and other medical devices. A supplier’s ability to provide documentation, traceability, and technical support further ensures that nitinol tubing meets the rigorous demands of medical applications.
Regulatory and Quality Standards
Medical Device Standards
Regulatory and quality standards play a vital role in the production of nitinol tubing for medical stents. These standards ensure that every device meets strict safety and performance requirements. Regulatory bodies such as the FDA and ISO set guidelines that manufacturers must follow throughout the entire production process. These guidelines cover biocompatibility, mechanical performance, and material purity for nitinol used in medical devices.
Compliance with medical standards confirms that nitinol tubing is safe and effective for use in healthcare.
The FDA and ISO provide comprehensive guidance on the manufacturing and testing of nitinol tubing.
ISO 13485 certification serves as a recognized quality management system standard for medical device manufacturers.
Manufacturers must maintain rigorous quality control and precise inspections to meet the high demands of medical applications.
Quality management systems help manufacturers track every step of production. These systems ensure that nitinol tubing for stents consistently meets regulatory expectations and supports patient safety.
Testing and Inspection
Testing and inspection protocols for nitinol tubing in medical stents require high precision and thorough documentation. Manufacturers use advanced tools such as laser micrometers and ultrasonic systems to measure outer diameter, inner diameter, and wall thickness. Strict size tolerances apply to each tubing batch, as shown in the table below:
OD Range (mm) | OD Tolerance (mm) | ID Tolerance (mm) |
|---|---|---|
≤ 0.3 | ±0.005 | ±0.010 |
0.3 to 0.5 | ±0.007 | ±0.015 |
0.5 to 1.5 | ±0.015 | ±0.020 |
1.5 to 2.5 | ±0.020 | ±0.030 |
2.5 to 3.5 | ±0.020 | ±0.040 |
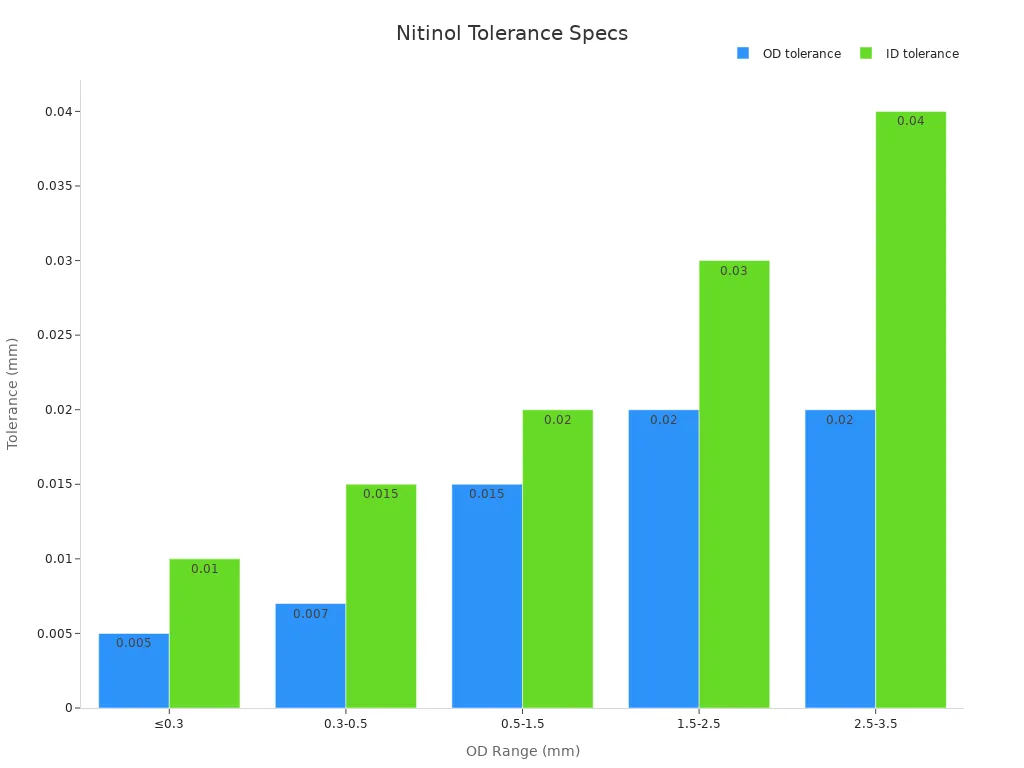
Manufacturers apply Statistical Process Control (SPC) techniques, including control charts and Pareto charts, to monitor production quality. The FDA guidance requires comprehensive test reporting. This includes statistical summaries such as minimum, maximum, mean, and standard deviation. Test protocols must be established before testing begins. These protocols should explain the rationale for each parameter and its clinical relevance. Manufacturers must provide raw data and detailed analysis to support claims of safety and effectiveness.
For stress and strain analysis, the FDA recommends computational modeling that considers device geometry, material properties, and validation against bench testing. Accelerated durability testing simulates up to ten years of use under physiological conditions. Manufacturers must justify sample sizes and test durations based on fatigue analysis. All loading conditions should reflect real clinical use, and any deviations must be explained.
Consistent quality in nitinol tubing ensures that medical stents perform reliably and safely in patients.
Superelastic nitinol tubing offers unmatched flexibility, durability, and biocompatibility for medical stents in stroke intervention. High manufacturing quality and strict regulatory compliance ensure that nitinol performs reliably in demanding medical environments. Medical professionals should use this knowledge to select nitinol tubing that meets the highest standards. Informed choices help improve patient outcomes and advance the field of medical devices.
FAQ
What makes nitinol tubing ideal for stroke intervention stents?
Nitinol tubing offers superelasticity and shape memory. These properties allow stents to expand and adapt inside blood vessels. Medical professionals value nitinol for its flexibility, durability, and biocompatibility.
How does superelasticity benefit stent performance?
Superelasticity lets nitinol stents recover their shape after bending or compression. This feature helps stents maintain vessel support and resist permanent deformation during and after placement.
Are there any risks associated with nitinol in the body?
Clinical studies show nitinol is safe for most patients.
Surface treatments reduce nickel ion release.
Allergic reactions remain rare.
Medical teams monitor patients for any adverse effects.
What quality standards must nitinol tubing meet for medical use?
Standard | Purpose |
|---|---|
ASTM F2063 | Material composition |
ISO 10993 | Biocompatibility testing |
ISO 13485 | Quality management systems |
Manufacturers must follow these standards to ensure safety and reliability.

