Step-by-Step Guide to Selecting Nitinol Tubing for Stents
Selecting the right nitinol tubing for stents and guidewires is critical for ensuring the safety and performance of medical devices. Nitinol, a shape memory alloy, stands out due to its unique properties, including biocompatibility, elasticity, and fatigue resistance. These characteristics make it indispensable in applications such as nitinol stents, guidewires, and neurovascular devices. The growing demand for minimally invasive treatment options further underscores the importance of nitinol in modern healthcare. Understanding application-specific needs, such as environmental conditions and mechanical requirements, is essential when choosing the right nitinol wire or tubing. This ensures optimal functionality and longevity of medical-grade devices.
Key Takeaways
Pick nitinol tubing based on what it will be used for. Think about flexibility, safety for the body, and strength for best results.
Look at important features like shape memory and bending ability. These are key for stents and guidewires to work well.
Follow rules like ASTM F2063 to meet safety standards. These rules help make medical devices safe and effective.
Talk to manufacturers about custom options. Special tubing can fit specific needs and improve how devices work for patients.
Test and check nitinol tubing carefully. This makes sure it is strong and reliable for long-term medical use.
Application Needs for Nitinol Tubing
Environmental and Biological Conditions
Nitinol tubing must perform reliably under diverse environmental and biological conditions. Medical devices like stents and guidewires often operate in challenging environments, including exposure to bodily fluids and varying temperatures. Nitinol, as a shape memory alloy (SMA), offers exceptional corrosion resistance, ensuring durability in such conditions. Its biocompatibility minimizes the risk of adverse reactions, making it ideal for neurovascular devices and other medical applications. Additionally, nitinol tubing must withstand chemical exposure and maintain its properties over time, ensuring the safety and longevity of medical devices.
Physical and Mechanical Requirements
The physical and mechanical properties of nitinol tubing play a crucial role in its selection for stents and guidewires. Key performance metrics include tensile strength, fatigue life, and residual elongation. For example, tensile strength ensures the tubing can endure the forces applied during deployment, while fatigue life determines its ability to withstand repeated stress cycles. The table below highlights some critical metrics:
Metric | Description |
|---|---|
Fatigue Life | Measures strain amplitudes and fracture probabilities over 10⁷ cycles. |
Assesses the maximum load the tubing can handle before failure. | |
Residual Elongation | Evaluates the difference in tensile strain between loading and unloading curves. |
These properties ensure nitinol tubing maintains its shape memory and superelasticity, critical for medical devices requiring precision and flexibility.
Regulatory and Compliance Considerations
Compliance with industry standards is essential when choosing the right nitinol wire or tubing for medical applications. Standards like ASTM F2063 outline the requirements for nitinol as a shape memory alloy, ensuring its safety and performance in medical devices. Manufacturers must also adhere to regulatory guidelines specific to their region, such as FDA regulations in the United States or CE marking in Europe. Ignoring these standards can lead to device failure or regulatory penalties, emphasizing the importance of thorough compliance checks during the development process.
Properties of Nitinol Tubing for Stents and Guidewires
Shape Memory and Superelasticity
Nitinol, as a shape memory alloy, exhibits two remarkable properties: shape memory and superelasticity. These characteristics make it indispensable in medical devices like stents and guidewires. Shape memory allows nitinol tubing to return to its original shape after deformation when exposed to specific temperatures. This property is particularly useful in self-expanding stents, which need to adapt to the dynamic environment of the human body. Superelasticity, on the other hand, enables nitinol to withstand significant strain without permanent deformation. This feature ensures that nitinol tubing can endure the mechanical stresses encountered during deployment and use.
Empirical studies highlight the importance of these properties in medical applications. For instance, nitinol's stress hysteresis enhances its biomechanical compatibility with body structures, while its superelasticity supports minimally invasive procedures. These attributes make nitinol tubing for stents and guidewires a preferred choice in modern healthcare.
Biocompatibility and Corrosion Resistance
Biocompatibility and corrosion resistance are critical for the long-term performance of nitinol tubing in medical devices. Nitinol's biocompatibility minimizes the risk of adverse reactions, ensuring its safe use in stents and other implants. Its exceptional corrosion resistance further enhances its suitability for prolonged exposure to bodily fluids. Research shows that nitinol performs well in MRI environments, making it ideal for imaging-compatible medical devices.
Studies also reveal that corrosion pits can initiate fatigue cracks, emphasizing the need for proper surface treatments like electropolishing and coating. These processes improve the material's resistance to corrosion and enhance its overall biocompatibility. By addressing these factors, manufacturers can ensure the reliability and safety of nitinol-based medical devices.
Durability and Kink Resistance
Durability and kink resistance are essential properties of nitinol tubing, particularly for stents and guidewires that operate in challenging environments. Nitinol's ability to resist fatigue and maintain its structural integrity under cyclic loads ensures its long-term performance. The FDA recommends accelerated durability testing to evaluate failure modes such as fretting, abrasion, and fracture. These tests simulate physiological conditions, providing insights into the material's real-world performance.
Kink resistance is another critical factor, especially for guidewires that navigate complex vascular pathways. Nitinol's superelasticity and unique mechanical properties allow it to bend without kinking, ensuring smooth navigation and reducing the risk of device failure. These attributes make nitinol tubing a reliable choice for demanding medical applications.
Types of Nitinol Tubing
Seamless vs. Welded Nitinol Tubing
Seamless nitinol tubing is manufactured without joints, offering superior uniformity and strength. This type is ideal for medical devices like stents and guidewires, where structural integrity is critical. Seamless tubing minimizes the risk of weak points, ensuring consistent performance under high stress. Welded nitinol tubing, on the other hand, is created by joining edges of a nitinol sheet. While it is more cost-effective, it may exhibit slight variations in mechanical properties due to the welding process.
The choice between seamless and welded tubing depends on the application. For instance, seamless tubing is preferred for high-precision stents, while welded tubing may suffice for less demanding medical applications. The global nitinol market, valued at $16.8 billion in 2023, reflects the growing demand for both types. North America leads consumption, driven by advanced healthcare infrastructure and a high prevalence of cardiovascular diseases.
Thin-Walled vs. Thick-Walled Tubing
Wall thickness significantly impacts the performance of nitinol tubing. Thin-walled tubing offers greater flexibility and is often used in minimally invasive medical devices. Its reduced thickness allows for easier navigation through complex anatomical pathways, making it ideal for neurovascular stents. Thick-walled tubing, however, provides enhanced durability and strength. It is better suited for applications requiring higher mechanical support, such as load-bearing implants.
Manufacturers must carefully evaluate the wall thickness based on the device's intended use. For example, thin-walled tubing is essential for guidewires navigating intricate vascular systems, while thick-walled tubing ensures the longevity of structural implants.
Custom vs. Standard Nitinol Tubing
Custom nitinol tubing is tailored to meet specific application requirements, offering precise control over dimensions, properties, and performance. This customization ensures optimal compatibility with unique medical devices. Standard tubing, however, is pre-manufactured with fixed specifications, making it a cost-effective option for general applications.
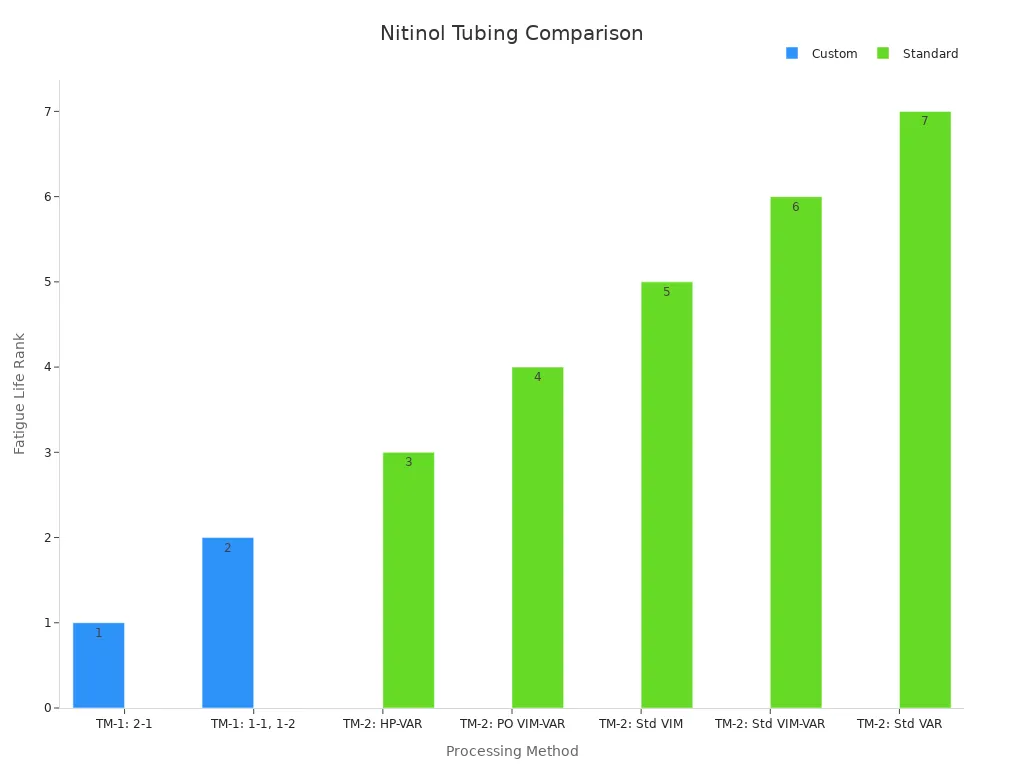
Clinical studies highlight the advantages of custom tubing in terms of fatigue life and performance. The table below illustrates the fatigue life rankings of various processing methods for nitinol tubing:
Processing Method | Fatigue Life Ranking | Notes |
|---|---|---|
TM-1: 2-1 | 1st | Superior fatigue life at all probabilities |
TM-1: 1-1, 1-2 | 2nd | Virtually identical fatigue life |
TM-2: HP-VAR | 3rd | Converges with TM-1: 1-1 and 1-2 at 10⁷ cycles |
TM-2: PO VIM-VAR | 4th | Slightly inferior to HP-VAR |
TM-2: Std VIM | 5th | Increasingly worse fatigue lives |
TM-2: Std VIM-VAR | 6th | Increasingly worse fatigue lives |
TM-2: Std VAR | 7th | Increasingly worse fatigue lives |
Custom tubing demonstrates superior fatigue life, making it indispensable for high-performance stents and guidewires. The choice between custom and standard tubing depends on the complexity and precision required for the medical device.
Step-by-Step Selection Guide
Define Application-Specific Needs
Defining application-specific needs is the first step in selecting nitinol tubing for stents and guidewires. Medical devices like stents must meet stringent requirements based on their intended use. For example, nitinol tubing used in blood vessels requires high flexibility and biocompatibility, while tubing for structural implants demands enhanced durability. The nitinol alloy composition plays a critical role in determining mechanical properties such as shape memory and superelasticity. Adjusting the ratio of nickel and titanium allows customization to meet unique patient needs.
Manufacturers must also consider dimensions like material thickness and length. For instance, stent production often requires nitinol tubing with a thickness of 0.40 mm and a cut length of 2,522 mm. Annual volume requirements, such as 5,000 pieces, further influence the selection process. Customization ensures the tubing aligns with the specific demands of medical applications, enhancing device performance and patient outcomes.
Evaluate Nitinol Tubing Properties
Evaluating nitinol tubing properties is essential for ensuring its suitability for medical devices. Key performance indicators include shape memory effect, superelasticity, biocompatibility, and corrosion resistance. Shape memory allows nitinol tubing to recover its original shape when heated above its transformation temperature, while superelasticity enables it to withstand deformation without permanent damage. These properties are critical for stents and guidewires that navigate complex anatomical pathways.
Research highlights tensile strength values ranging from 500 MPa to 900 MPa for nitinol tubing. Process-optimized tubes can achieve a maximum local strain of up to 6%, demonstrating their ability to endure significant stress. Manufacturing techniques, material composition, and thermomechanical processing further enhance these properties. Surface characteristics, such as titanium oxides, improve durability and corrosion resistance, ensuring the tubing performs reliably in demanding environments.
Property | Description |
|---|---|
Shape Memory Effect | Recovers its original shape when heated above its transformation temperature. |
Superelasticity | Returns to its original shape after deformation without permanent damage. |
Biocompatibility | Non-toxic and well-tolerated by the human body. |
Corrosion Resistance | Resists corrosion, enhancing longevity in medical and industrial applications. |
Consult Experts and Manufacturers
Consulting experts and manufacturers is a crucial step in selecting nitinol tubing for stents and guidewires. Suppliers should offer customization options for tube sizes, thicknesses, and finishes to meet specific medical application needs. Advanced manufacturing tools ensure the tubing maintains smoothness, strength, and uniformity in performance.
A step-by-step methodology aids in identifying reliable suppliers:
Research Supplier Reputation: Read reviews and testimonials to assess reliability and product quality.
Request Samples and Prototypes: Test product quality before committing to a supplier.
Visit the Supplier's Facility: Observe production processes and quality control measures.
Compare Different Suppliers: Make a list of important factors and compare suppliers side by side.
Follow Medical Device Standards: Ensure compliance with safety and precision standards for medical tools.
Customization remains vital for achieving optimal performance. Suppliers should provide tubing tailored to the specific requirements of medical devices, ensuring safety and efficiency in healthcare applications.
Test and Validate Tubing Performance
Testing and validating nitinol tubing performance is a critical step in ensuring its reliability for medical devices like stents and guidewires. Proper evaluation guarantees that the tubing meets the stringent demands of medical applications, including flexibility, durability, and biocompatibility.
Performance testing often involves advanced simulation tools and standardized protocols. For instance, Midas NFX software evaluates the elasticity and adaptability of nitinol stents under large deformations. Flat-type and three-point bending tests assess superelasticity, a key property for stents navigating complex vascular pathways. Mechanical properties such as an elastic modulus of 28 GPa, a Poisson’s ratio of 0.3, and a tensile strength of 100 MPa are commonly measured to ensure the tubing can withstand physiological stresses.
Flexibility testing is another essential aspect of validation. Following ASTM F2606-08 standards, researchers compare the flexibility of different stent designs. Results have shown that Solitaire FR stents exhibit superior flexibility (0.38 ± 0.11 N) compared to Trevo XP ProVue (0.91 ± 0.11 N) and Stent D (0.59 ± 0.05 N). This highlights the importance of selecting nitinol tubing with optimal mechanical properties for specific medical applications. Stent D, for example, demonstrated promising performance in vascular phantom models, combining mechanical strength with a high thrombus retrieval rate.
Validation also includes accelerated durability testing to simulate long-term use. These tests identify potential failure modes, such as fatigue or corrosion, ensuring the tubing maintains its structural integrity over time. Manufacturers must conduct thorough evaluations to confirm that nitinol tubing meets the rigorous demands of stents and other medical devices, ultimately enhancing patient safety and device performance.
Avoiding Common Mistakes
Ignoring Compliance Standards
Compliance standards play a critical role in ensuring the safety and effectiveness of medical devices like stents. Overlooking these standards can lead to regulatory penalties, product recalls, or even patient harm. Organizations such as the FDA and ISO have established stringent guidelines for nitinol tubing used in medical applications. For instance, ASTM F2063 specifies the chemical composition and mechanical properties required for nitinol as a shape memory alloy. Manufacturers must adhere to these standards to ensure the tubing meets biocompatibility and performance requirements.
Ignoring compliance can result in devices failing under physiological conditions. For example, stents that do not meet corrosion resistance standards may degrade prematurely, leading to complications. Thorough documentation and testing are essential to demonstrate adherence to these regulations. Collaborating with experienced manufacturers who understand these requirements can help avoid costly mistakes.
Selecting the Wrong Tubing Type
Choosing the wrong type of nitinol tubing can compromise the performance and longevity of stents. Factors such as wall thickness, manufacturing method, and material purity significantly impact the tubing's suitability for specific applications. For instance, thin-walled tubing offers flexibility for neurovascular stents, while thick-walled tubing provides the durability needed for load-bearing implants.
The consequences of selecting inappropriate tubing are well-documented. The table below highlights common issues:
Evidence Type | Description |
|---|---|
Documented Cases | Fatigue fractures in clinical settings due to lower purity materials leading to premature failures in medical devices. |
Performance Statistics | High purity nitinol material significantly improves durability performance, with specific applications requiring materials that can withstand millions of cycles without failure. |
Risk Assessment | Fracture risk is linked to the presence of inclusions or voids in the material, which can initiate fractures under stress, emphasizing the need for high purity materials. |
High-purity nitinol tubing minimizes the risk of fractures and ensures the stents perform reliably over time. Manufacturers must carefully evaluate the tubing type based on the device's intended use to avoid these pitfalls.
Overlooking Long-Term Durability
Long-term durability is a critical consideration for stents, as these devices must endure years of physiological stress without failure. Overlooking this factor can lead to device fatigue, fractures, or corrosion, compromising patient safety. Nitinol tubing's durability depends on its fatigue resistance, surface finish, and material composition.
Accelerated durability testing helps simulate real-world conditions to identify potential failure modes. For example, stents must withstand millions of cycles of deformation without losing structural integrity. Surface treatments like electropolishing enhance corrosion resistance, further extending the device's lifespan. Manufacturers must prioritize durability during the selection process to ensure the tubing meets the demands of long-term medical applications.
Selecting the right nitinol tubing for stents and guidewires ensures optimal device performance and patient safety. Application-specific requirements, such as mechanical properties and biocompatibility, must guide the selection process. Manufacturers play a pivotal role in tailoring tubing solutions to meet these needs. Research highlights the impact of manufacturing techniques on mechanical properties and fatigue life, as shown below:
Key Findings | Description |
|---|---|
Impact of Manufacturing Techniques | Different techniques significantly affect the mechanical properties of nitinol tubing, crucial for medical applications. |
Fatigue Life Correlation | The study indicates a strong correlation between tube processing methods and the fatigue life of nitinol components. |
Collaborating with manufacturers ensures precision and reliability in nitinol tubing, enhancing the performance of stents and other medical devices.
FAQ
What is the primary advantage of using nitinol tubing in stents?
Nitinol tubing offers exceptional shape memory and superelasticity, enabling stents to adapt to the dynamic environment of the human body. These properties ensure precise deployment and long-term functionality, making nitinol an ideal material for medical devices requiring flexibility and durability.
How does wall thickness affect nitinol tubing performance?
Wall thickness significantly impacts flexibility and strength. Thin-walled tubing enhances maneuverability in minimally invasive procedures, while thick-walled tubing provides greater durability for load-bearing applications. Selecting the appropriate thickness ensures optimal performance based on the device's intended use.
Are there specific standards for nitinol tubing in medical devices?
Yes, standards like ASTM F2063 define the chemical composition and mechanical properties of nitinol for medical applications. Adhering to these standards ensures biocompatibility, corrosion resistance, and safety, which are critical for stents and guidewires.
Can nitinol tubing be customized for unique medical applications?
Manufacturers offer customization options for nitinol tubing, including dimensions, alloy composition, and surface finishes. Customization ensures compatibility with specific medical devices, enhancing performance and meeting unique application requirements.
How can manufacturers ensure the durability of nitinol tubing?
Manufacturers conduct accelerated durability testing to simulate long-term use. Techniques like electropolishing improve corrosion resistance, while fatigue testing ensures the tubing withstands repeated stress cycles. These measures guarantee the tubing's reliability in demanding medical environments.
See Also
A Comprehensive Approach to Choosing Nitinol Tubing Effectively
Detailed Instructions for Creating Nitinol Microtubing in Neurovascular Use
Guidelines for Choosing the Ideal Nitinol Tubing Supplier
Understanding the Manufacturing Process of Nitinol Tubing for Medicine
Investigating Nitinol Tubing Uses in Medical Device Technology

