How Precision is Achieved in Nitinol Tubing for Stents
Precision plays a vital role in the creation of nitinol tubing for medical stents. Accurate manufacturing ensures that stents perform effectively in the human body, maintaining structural integrity while navigating complex blood vessels. Nitinol, a unique alloy known for its superelasticity and shape memory, allows stents to adapt to intricate anatomical structures. This adaptability minimizes the risk of complications and enhances patient outcomes. Additionally, nitinol exhibits excellent biocompatibility and corrosion resistance, which contribute to its widespread use in life-saving medical devices like stents.
Key Takeaways
Nitinol tubing is important for stents because it bends easily and remembers its shape. This helps stents fit blood vessels and keep blood flowing.
Nitinol is safe for the body and doesn’t rust, lowering the chance of problems. This makes it a good choice for medical tools.
Special methods like laser cutting and tiny machining make sure the tubing is the right size. This is key for stents to work well.
Careful testing and following medical rules ensure nitinol tubing is safe and reliable for medical use.
Unique Properties of Nitinol for Stents
Superelasticity and Shape Memory Effect
Nitinol's superelasticity and shape memory effect make it an ideal material for stents. These properties allow nitinol to return to its original shape after deformation, ensuring that stents can expand and conform to the shape of blood vessels. This adaptability is crucial for maintaining blood flow in complex anatomical structures.
Scientific studies have demonstrated the remarkable transformation behavior of nitinol. For instance:
The transformation temperature (Ms) in nitinol's welded joints increases by 10 K.
The shape memory function decreases significantly under varying strain conditions.
These findings highlight nitinol's ability to perform reliably under physiological conditions, making it a preferred choice for medical applications.
Biocompatibility and Corrosion Resistance
Biocompatibility and corrosion resistance are critical for any material used in implantable devices. Nitinol excels in both areas. When exposed to air, nitinol forms a stable titanium oxide layer that prevents nickel ion release. This protective barrier enhances its biocompatibility and reduces the risk of adverse reactions in the body.
Additional studies confirm nitinol's suitability for medical use:
In vitro and in vivo tests show minimal cytotoxicity and negligible inflammatory responses.
Electropolishing techniques further reduce nickel leaching, ensuring long-term safety.
The titanium oxide layer's stability is essential for maintaining corrosion resistance, even in challenging physiological environments.
These properties make nitinol a reliable material for stents, ensuring durability and patient safety.
Strength and Flexibility for Medical Applications
Nitinol combines exceptional strength with flexibility, enabling stents to withstand the dynamic forces within blood vessels. Performance metrics validate its superior mechanical properties. For example:
Stent Model | Flexibility (N) | Ranking of Flexibility |
|---|---|---|
Solitaire FR | 0.38 ± 0.11 | Highest flexibility |
Stent D | 0.59 ± 0.05 | Moderate flexibility |
Trevo XP ProVue | 0.91 ± 0.11 | Lowest flexibility |
Moreover, nitinol tubing retains its shape memory and superelasticity over 10 million cycles, demonstrating unparalleled fatigue resistance. The SUPERA wire-interwoven nitinol stent, for instance, showed only a 0.03-inch displacement under a compressive force of 4 lbs, compared to 0.2 inches for laser-cut stents. This performance underscores nitinol's ability to maintain structural integrity under stress, ensuring reliable functionality in medical applications.
Manufacturing Process of Nitinol Tubing for Medical Stents
Raw Material Preparation and Alloy Composition
The manufacturing process of nitinol tubing begins with the careful preparation of raw materials. Nitinol, an alloy of nickel and titanium, requires precise control over its composition to achieve its unique shape memory properties. Manufacturers melt nickel and titanium together to form a strong and uniform alloy. This step often employs advanced techniques like Vacuum Induction Melting (VIM) and Vacuum Arc Remelting (VAR).
Vacuum Induction Melting (VIM) prevents contamination by melting the metals in a controlled vacuum environment.
Vacuum Arc Remelting (VAR) further refines the alloy by removing impurities, ensuring consistency in its microstructure.
Step | Description |
|---|---|
Melting | Nickel and titanium are melted together to form a strong alloy. |
Vacuum Induction Melting (VIM) | Prevents contamination and maintains material purity. |
Vacuum Arc Remelting (VAR) | Refines the alloy by removing impurities and ensuring consistency. |
Dimensional accuracy testing ensures that the alloy meets precise specifications for medical devices. Functional property verification evaluates its performance benchmarks, while compliance with medical standards guarantees safety and reliability. These steps ensure that the nitinol alloy is ready for the next stages of manufacturing.
Shaping and Forming the Tubing
Once the alloy is prepared, the next step involves shaping and forming the tubing. This stage transforms the raw nitinol alloy into the tubular structures required for stents. Manufacturers use specialized techniques to achieve the desired dimensions and mechanical properties.
Shaping processes often adhere to strict industry standards, such as ASTM F2063, which specifies the properties of nickel-titanium alloys, including tensile strength and fatigue resistance. Additionally, ISO 10993 ensures biocompatibility testing, confirming the material's safety for medical applications.
Key performance tests validate the tubing's quality:
Tensile Strength Tests assess the tubing's ability to withstand mechanical stress.
Thermal Analysis evaluates phase transformation behavior, critical for achieving shape memory properties.
Cyclic Testing, required by the FDA, ensures that stents can endure extensive pulsatile loading over time.
These rigorous standards and tests ensure that nitinol tubing manufacturing produces reliable and high-performing medical devices.
Heat Treatment for Shape Memory and Superelasticity
Heat treatment plays a crucial role in unlocking nitinol's shape memory properties and superelasticity. This process involves heating the tubing to specific temperatures to optimize its microstructure. The formation of Ni4Ti3 precipitates during aging enhances superelasticity by increasing the stress required for plastic deformation. This adjustment favors the formation of stress-induced martensite, which is essential for achieving the desired mechanical behavior.
Controlled experiments have demonstrated the effectiveness of heat treatment in improving nitinol's properties. For example:
Treatment Condition | |
|---|---|
As-built | 2.2 ± 0.5 |
S 400 °C | 0.3 ± 0.2 |
S 500 °C | 0.4 ± 0.1 |
DA 400 °C | 0.6 ± 0.1 |
DA 500 °C | 0.9 ± 0.6 |
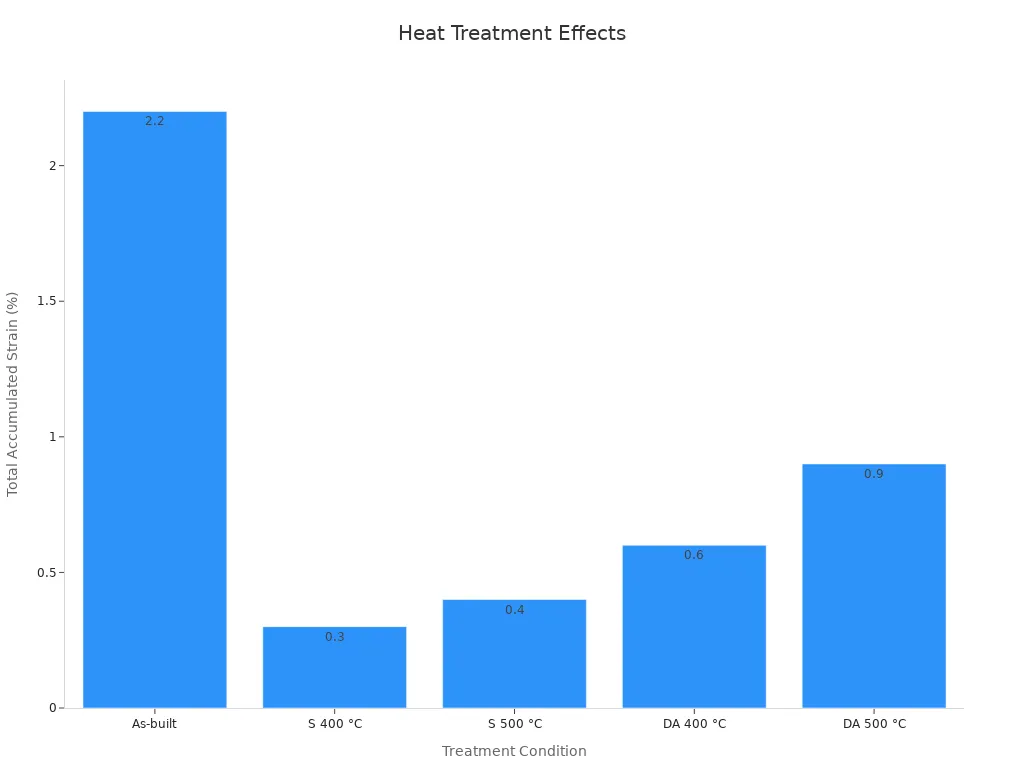
Strain recovery of 5.5% and a recovery ratio of 95% have been observed after aging at 600 °C for 1.5 hours without solution annealing. These results highlight the importance of precise heat treatment in achieving the exceptional performance required for nitinol tubing in stents.
Surface finishing and electropolishing for smoothness
Surface finishing and electropolishing are critical steps in the production of nitinol tubing for medical applications. These processes refine the surface of the tubing, ensuring it meets the stringent requirements for use in stents. A smooth surface is essential for reducing friction during insertion and minimizing the risk of complications within the body.
Electropolishing, a widely used technique, removes surface irregularities and creates a uniform finish. This process involves immersing the nitinol tubing in an electrolytic solution and applying an electric current. The current selectively dissolves microscopic peaks on the surface, leaving behind a polished and defect-free finish. This step not only enhances the tubing's appearance but also improves its performance and reliability.
The benefits of electropolishing extend beyond aesthetics. It significantly reduces the thickness of the oxide layer on nitinol, which lowers nickel release and enhances corrosion resistance. This improvement contributes to the biocompatibility of the tubing, making it safer for long-term implantation. Additionally, electropolished surfaces exhibit lower protein adsorption, which suggests improved hemocompatibility. This property is crucial for reducing the risk of blood clot formation, a common concern with implantable devices.
The following table highlights how surface finishing and electropolishing contribute to the smoothness and reliability of nitinol tubing:
Evidence Description | Contribution to Smoothness and Reliability |
|---|---|
Enhances biocompatibility and reliability of nitinol tubing. | |
In vitro evaluation shows lower protein adsorption in electropolished samples. | Suggests improved hemocompatibility, contributing to reliability. |
Lower thrombotic response in electropolished samples compared to native Nitinol. | Indicates enhanced hemocompatibility, supporting reliability. |
Electropolishing eliminates surface defects that could compromise performance. | Ensures safety and reliability of medical tubing. |
Surface treatments create smoother surfaces, reducing friction during insertion. | Improves device insertion and overall performance. |
Surface finishing also plays a vital role in ensuring the tubing's mechanical integrity. By eliminating surface defects, manufacturers reduce the likelihood of stress concentrations that could lead to fractures or failures. This step ensures that the tubing maintains its structural integrity under the dynamic forces it encounters within the body.
Techniques for Achieving Precision in Nitinol Tubing
Laser Cutting and Micromachining
Laser cutting and micromachining are essential techniques for achieving dimensional precision in superelastic nitinol tubing. These methods utilize high-powered lasers and advanced micromachining tools to create intricate designs and precise cuts required for medical applications. The laser's focused beam ensures minimal thermal distortion, preserving the tubing's mechanical properties and structural integrity.
Micromachining complements laser cutting by enabling the creation of complex geometries and ultra-fine features. This technique is particularly useful for stents, where precise patterns and openings are critical for optimal performance. Real-time monitoring systems enhance these processes by detecting variations as small as ±5 µm, ensuring consistent quality throughout production.
Laser cutting and micromachining not only improve dimensional precision but also reduce material waste, making them cost-effective solutions for manufacturing precision tubing.
Dimensional Accuracy and Tolerances
Dimensional accuracy is a cornerstone of precision tubing manufacturing. Tight tolerances ensure that nitinol tubing meets the exact specifications required for medical applications. Manufacturers employ advanced techniques like laser micrometry and ultrasonic testing to validate dimensional tolerances and detect internal flaws. These methods guarantee that the tubing's outer diameter, wall thickness, and length remain within strict limits.
The following table highlights the dimensional specifications achieved in nitinol tubing manufacturing:
Measurement Type | Specification |
|---|---|
Outer Diameter | Standard 1.61mm |
Wall Thickness | Ranges from 0.1mm to 15mm |
Length | Up to 6000mm |
Tolerance Control | Tight tolerance control |
Inspection Protocols | Rigorous for dimensional stability |
Electropolishing further refines dimensional accuracy by eliminating surface defects and ensuring smoothness. Non-destructive testing (NDT) methods, such as ultrasonic and X-ray inspections, play a vital role in detecting internal flaws and verifying the tubing's structural integrity. These rigorous protocols ensure that superelastic nitinol tubing meets the high standards required for medical applications.
Advanced Tooling and Automation
Advanced tooling and automation have revolutionized the production of precision tubing. Automated systems equipped with real-time monitoring and feedback mechanisms enable manufacturers to achieve tolerances as low as ±0.0005 inches. These systems ensure consistent quality and reduce human error, enhancing the reliability of nitinol tubing for medical applications.
Automation also improves surface quality by reducing roughness, which enhances fatigue resistance and extends the tubing's lifespan. Customizable tooling allows manufacturers to tailor tubing dimensions and properties to specific requirements, ensuring compatibility with diverse stent designs. The table below outlines key improvements achieved through advanced tooling:
Improvement Type | Details |
|---|---|
Achieved tolerances as low as ±0.0005 inches | |
Improved Surface Quality | Reduced surface roughness for better fatigue resistance |
Customizability | Tailored tubing to specific requirements |
Cold working and heat treatment processes further enhance the tubing's superelasticity and shape memory properties, ensuring optimal performance in medical applications. These advancements underscore the importance of automation and tooling in achieving dimensional precision and reliability in nitinol tubing.
Quality Control in Nitinol Tubing for Stents
Testing for Mechanical Properties and Biocompatibility
Mechanical testing and biocompatibility testing are essential components of quality control in nitinol tubing production. These tests ensure that the tubing meets the stringent requirements for medical devices. Mechanical testing evaluates the tubing's ability to withstand stress, fatigue, and deformation under physiological conditions. For example, tensile strength assessments and thermal analysis validate the material's performance and alignment with medical standards.
Biocompatibility testing confirms that nitinol tubing is safe for implantation. Studies have shown minimal tissue reactions, with histological evaluations scoring between 0 and 1. Additionally, no significant inflammatory responses or necrosis were observed in tested samples. These results demonstrate the tubing's compatibility with human tissues, ensuring its reliability in medical applications.
Evidence Type | Description |
|---|---|
Tissue Reactions | Minimal reactions post-implantation, with scores ranging from 0 to 1. |
Intraocular Pressure Reduction | Sustained 30% reduction in intraocular pressure over six months. |
Histological Findings | No significant inflammatory responses or necrosis observed. |
Compliance with Medical-Grade Standards (e.g., ISO, ASTM)
Compliance with medical-grade standards is critical for ensuring the quality and safety of nitinol tubing. Certifications like ASTM F2063 and ISO 13485 validate that the tubing meets rigorous testing and regulatory requirements. ASTM F2063 ensures the material's performance criteria, including tensile strength and fatigue resistance. ISO 13485 demonstrates adherence to quality management systems specific to medical devices, while ISO 9001 confirms a supplier's commitment to consistent quality.
Certification Type | Importance in Quality Assurance |
|---|---|
ASTM F2063 | Validates material performance criteria for medical-grade nitinol. |
ISO 9001 | Confirms a supplier’s commitment to quality and compliance. |
ISO 13485 | Ensures adherence to regulatory standards in medical device manufacturing. |
These certifications provide documented evidence of compliance, ensuring that nitinol tubing is safe and reliable for use in stents and other medical devices.
Inspection Methods (e.g., Visual, Ultrasonic, and X-ray)
Inspection methods play a vital role in detecting defects and ensuring the structural integrity of nitinol tubing. Visual testing (VT) is the most commonly used method, allowing inspectors to identify surface discontinuities during multiple production stages. Ultrasonic testing (UT) uses high-frequency sound waves to detect internal inconsistencies, making it ideal for raw material inspections. Radiographic testing (RT), which employs X-rays, provides detailed images of internal structures, ensuring the tubing's reliability.
Inspection Method | Description | Application in Nitinol Stents |
|---|---|---|
Ultrasonic Testing | Uses high-frequency ultrasonic energy to detect inconsistencies and flaws. | Extensively used for raw material inspections and final testing. |
Visual Testing | Involves visual observation to evaluate surface discontinuities. | Commonly used for inspecting parts in multiple production stages. |
Radiographic Testing | Uses X-rays to capture images of internal structures. | Applied in raw materials and finished devices for internal inspection. |
Advanced imaging techniques, such as CT scans, further enhance inspection capabilities. For example, 4-slice CT imaging has shown high sensitivity in detecting in-stent restenosis (ISR), with detection rates ranging from 54% to 100%. These methods ensure that superelastic nitinol tubing meets the high-quality standards required for medical devices.
Precision in nitinol tubing production stems from its unique properties and advanced manufacturing techniques. Nitinol’s biocompatibility and elasticity make it indispensable for medical applications. Precision enhances stent durability and effectiveness, improving patient outcomes. Techniques like topology optimization and stress-free lattice designs further boost fatigue life and anchoring performance, reducing risks such as failure or endoleaks.
Quality control ensures that every stent meets stringent medical standards. Rigorous testing and compliance protocols validate mechanical properties and biocompatibility, guaranteeing reliability. Precision in nitinol tubing not only advances stent performance but also drives innovation in medical technology, offering safer and more effective solutions for patients worldwide.
FAQ
What makes nitinol tubing ideal for medical stents?
Nitinol tubing offers superelasticity, shape memory, and biocompatibility. These properties allow stents to adapt to blood vessels while maintaining structural integrity. Its corrosion resistance ensures durability, making it a reliable material for medical applications like neurovascular stents.
How does precision impact neurovascular stents?
Precision ensures that neurovascular stents fit accurately within blood vessels. This accuracy improves blood flow and reduces complications. Advanced manufacturing techniques, such as laser cutting, achieve the required dimensional tolerances for optimal performance.
What role does heat treatment play in nitinol tubing production?
Heat treatment enhances nitinol tubing's shape memory and superelasticity. It optimizes the alloy's microstructure, enabling it to recover its original shape after deformation. This process is essential for creating innovative medical devices like stents.
Why is surface finishing important for nitinol tubing?
Surface finishing improves the smoothness and biocompatibility of nitinol tubing. Electropolishing removes surface defects, reduces nickel release, and enhances corrosion resistance. These improvements ensure the tubing's safety and reliability in medical applications.
How is quality control maintained in nitinol tubing manufacturing?
Quality control involves mechanical testing, biocompatibility assessments, and compliance with standards like ASTM and ISO. Inspection methods, including ultrasonic and X-ray testing, detect defects and ensure the tubing meets medical-grade requirements.
See Also
The Manufacturing Process of Nitinol Tubing in Medicine
Nitinol Tubing's Impact on the Future of Medical Devices
Nitinol Tubing's Contribution to Progress in Medical Technology
Evaluating Nitinol Tubing's Strength Against Stainless Steel

