Why Nitinol Tubing Shape Memory Effect Mechanism Never Forgets Its Shape

You might wonder how nitinol tubing never forgets its original shape. The secret sits in its atomic structure, where atoms shift like pieces in a puzzle. When you bend nitinol, the memory inside does not disappear. Instead, temperature changes trigger a reversible phase change, shifting between martensite and austenite. Scientists use X-ray microdiffraction and digital image correlation to measure how nitinol’s memory and shape memory effect work under stress. These studies show that the Nitinol tubing shape memory effect mechanism relies on atoms moving together, not breaking apart. This makes nitinol a standout among shape memory materials.
Key Takeaways
Nitinol tubing remembers its shape because its atoms shift together without breaking, changing structure with temperature.
The shape memory effect comes from switching between two phases: soft martensite at low temperatures and stiff austenite at high temperatures.
The transition temperature and nickel-titanium ratio control when and how nitinol tubing returns to its original shape.
Nitinol tubing can bend and recover many times without losing its memory, making it ideal for medical and industrial uses.
Its unique atomic movement and fast, diffusionless phase changes let nitinol tubing respond quickly and reliably to heat and stress.
Shape Memory Effect in Nitinol

Atomic Structure Basics
When you look at nitinol, you see a metal that seems ordinary. Inside, though, its atoms form a unique crystal structure. This structure gives nitinol its famous shape memory effect. The atoms arrange themselves in patterns that can change with temperature. You can think of these atoms like puzzle pieces that fit together in different ways. When you cool or heat nitinol, the atomic bonds shift, but the atoms do not break apart. This movement lets nitinol tubing remember its original shape.
Scientists use X-ray Diffraction (XRD) to study these atomic changes. They measure how the lattice spacing changes as nitinol goes through a transition. These experiments show that the atomic structure can stretch or shrink, but the overall pattern stays the same. This is why nitinol, as a shape memory alloy, can return to its programmed shape after bending.
The shape memory effect depends on the way nitinol’s atoms move together. You see this effect most clearly when the tubing changes from one phase to another. The transition between phases happens at certain temperatures. The table below shows some important numbers about these transitions and the properties of nitinol:
Parameter | Value | Description |
|---|---|---|
Martensite start temperature | 43 °C | Temperature where martensite phase begins |
Martensite finish temperature | 32 °C | Temperature where martensite phase ends |
Young's modulus (Austenite) | 69 ± 1 GPa | Elastic modulus in high-temperature phase |
Young's modulus (Martensite) | 26 ± 1 GPa | Elastic modulus in low-temperature phase |
Logistic function midpoint (Tm) | 38.3 °C | Midpoint temperature of elastic property change |
Logistic function slope (k) | -1.4 (°C)^-1 | Rate of change of elastic properties with temperature |
Note: These numbers show how nitinol’s atomic bonds and memory change with temperature. The transition temperatures control when the shape memory effect happens.
Martensite vs. Austenite
Nitinol’s shape memory effect comes from its ability to switch between two main phases: martensite and austenite. Each phase has a unique crystal structure. When nitinol is cool, it enters the martensite phase. When you heat it, it changes to the austenite phase. This transition is what gives nitinol its memory.
Here is a table that compares the two phases:
Aspect | Austenite Phase | Martensite Phase |
|---|---|---|
Crystal Structure | Body-Centered Cubic (BCC) | Monoclinic |
Number of Crystallographic Variants | Single variant | Up to 24 variants |
Elastic Modulus / Stiffness | Higher stiffness | Lower stiffness |
Phase Transformation Nature | Stable at higher temperatures | Stable at lower temperatures |
Stress–Strain Behavior | Follows Hooke's law | Two yield regions, lower stiffness |
Transformation Mechanism | Diffusionless, shear transformation | Diffusionless, lattice distortion |
Mechanical Properties Impact | Higher stiffness and hardness | Rapid decrease in stiffness |
You can see that the transition between martensite and austenite changes how nitinol behaves. In the martensite phase, nitinol bends easily. In the austenite phase, it becomes stiff and snaps back to its original shape. This is the heart of the shape memory effect in nitinol tubing.
Scientific studies show that even small changes in the nickel-titanium ratio can shift the transition temperature by over 100°C. This means you can fine-tune nitinol’s memory and effect for different uses. The unique crystal structure of nitinol allows it to act as one of the most reliable shape memory alloys. By enhancing nitinol shape memory alloys, engineers create tubing that never forgets its shape, even after many cycles of bending and heating. This makes nitinol stand out among all shape memory materials.
Nitinol Tubing Shape Memory Effect Mechanism
Atomic Rearrangement
When you look at nitinol tubing, you see a metal that can bend and return to its original shape. The secret behind this lies in the nitinol tubing shape memory effect mechanism. At the atomic level, nitinol atoms act like a team of dancers. Each atom knows its place in the dance. When you bend the tubing, the atoms shift positions, but they do not break apart. Instead, they slide into new spots, keeping the overall pattern of the crystal lattice.
Imagine a group of puzzle pieces that fit together in more than one way. When you apply heat or cold, the pieces move to form a new picture, but the edges still match. This is how the shape memory effect works in nitinol. The atoms rearrange themselves during the transition between phases, but the basic structure stays the same. This allows the tubing to "remember" its original shape and return to it when the temperature changes.
Scientists have studied other materials that show similar behavior. For example, research on Ge2Sb2Te5 phase change materials used advanced x-ray methods to reveal how atoms rearrange during a transition. The study found that some atoms help the structure change, while others control how easily the change happens. Even as the material shifts from one phase to another, the lattice structure remains preserved. Another study used electron microscopy to watch nanoparticles change phase. The core structure stayed the same, even as the atoms moved to new positions. These findings support the idea that nitinol's memory comes from atomic rearrangement and lattice preservation.
Note: The nitinol tubing shape memory effect mechanism depends on the ability of atoms to move together in a coordinated way. This movement lets the tubing recover its shape again and again.
Diffusionless Transformation
The nitinol tubing shape memory effect mechanism relies on a special kind of phase change called a diffusionless transformation. In most metals, atoms move slowly from one place to another during a phase change. In nitinol, the atoms shift quickly and do not need to travel long distances. This is what makes the shape memory effect so unique.
The transition from austenite to martensite, and back, happens without atoms diffusing through the metal.
Instead, the atoms shuffle into new positions, changing the crystal structure in a snap.
The austenitic transformation changes the lattice from a simple cubic to a more complex shape, but the atoms stay close to their original spots.
Scientists have used X-ray and electron studies to show that these transitions involve electronic changes and lattice distortions, not atomic diffusion.
The strains from the transformation stay local, and the martensitic transformation packs the atoms more tightly, all without breaking the lattice.
This diffusionless process is why nitinol tubing can switch between shapes so quickly. The nitinol tubing shape memory effect mechanism allows the tubing to respond to temperature or stress almost instantly. You see this in the superelasticity and shape memory effect that make nitinol tubing so valuable in many fields.
The nitinol tubing shape memory effect mechanism stands out among shape memory alloys. The memory comes from the way atoms move together, not from atoms leaving their places. The transition between phases happens smoothly, and the tubing always returns to its programmed shape. This makes nitinol a leader in shape memory alloys and explains why its memory never fades.
Nitinol Transition Temperature and Atomic Bonds
Role of Transition Temperature
You can think of the nitinol transition temperature as the switch that controls the shape memory effect. When you heat or cool nitinol tubing, the temperature moves past a certain point. This point is the nitinol transition temperature. At this moment, the atoms inside the tubing shift from one phase to another. The transition from martensite to austenite, or back, happens because the nitinol transition temperature acts as a trigger.
When you stretch nitinol tubing, the phase front moves through the metal. The number and speed of these phase fronts depend on how fast you load the tubing and how much heat the tubing absorbs or releases. Scientists found that the nitinol transition temperature, together with the Clausius–Clapeyron relation, links the stress needed for the transition to the temperature at the phase front. This means that the nitinol transition temperature controls when and how the tubing changes shape.
You can see the effect of different nitinol transition temperatures in shape recovery performance. The table below shows how programming temperature changes the recovery ratio and fixation ratio for different fiber types:
Fiber Type | Programming Temperature (°C) | Deformation Strain (%) | Fixation Ratio (R_f, %) | Recovery Ratio (R_r, %) |
|---|---|---|---|---|
Random | 37 | 100 | ~96.4 | ~75.4 |
Random | 46 | 100 | ~97.9 | ~71.1 |
Aligned | 37 | 100 | ~95.6 | ~75.4 |
Aligned | 46 | 100 | ~96.9 | ~70.7 |
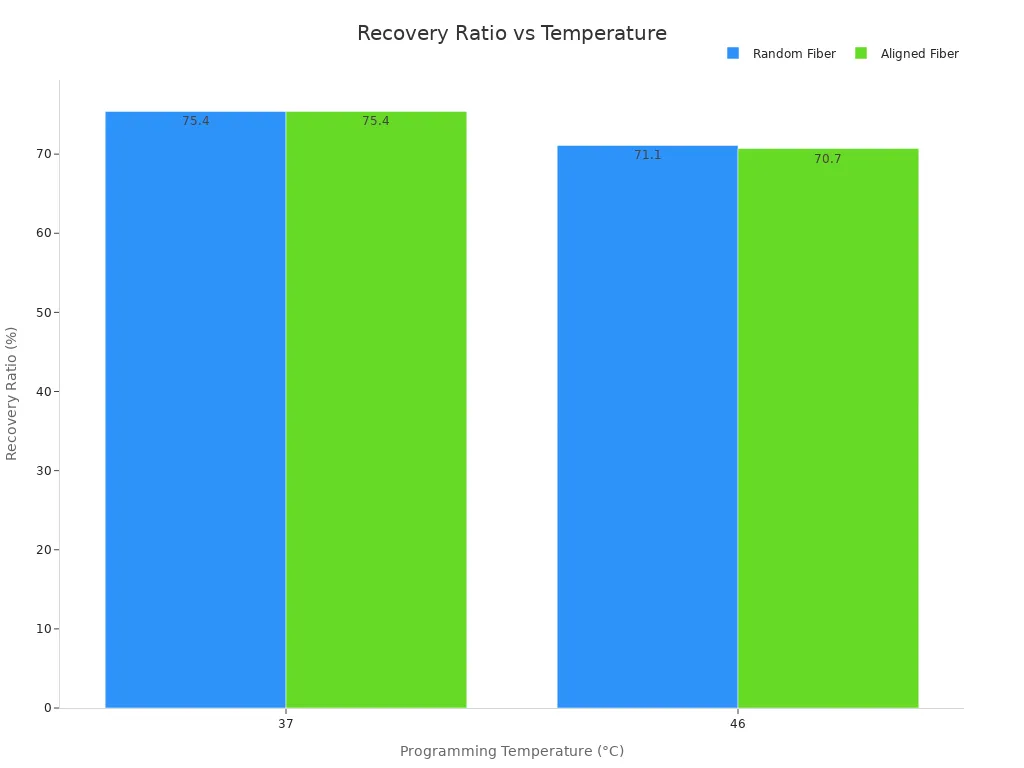
Dynamic mechanical analysis shows that aligned fibers recover faster than random fibers at the same nitinol transition temperature. When you increase the programming temperature, the recovery ratio drops. This proves that the nitinol transition temperature has a strong impact on how well the tubing returns to its original shape.
You can also find different effective temperature ranges for shape memory behavior in various materials. For example, commercial Ni–Ti alloys work best between 273 K and 353 K. The austenite finish temperature is a key part of this range, as it marks when the transition to austenite completes.
Nickel-Titanium Ratio
You can fine-tune the nitinol transition temperature by changing the nickel-titanium ratio. Even a small change in this ratio can shift the transition temperature by many degrees. If you add more nickel, the nitinol transition temperature drops. If you lose nickel, maybe because titanium bonds with oxygen or carbon, the nitinol transition temperature rises. The austenite finish temperature also changes with the nickel-titanium ratio.
The thermal hysteresis, or the temperature gap between the forward and reverse transition, depends on the exact nickel-titanium ratio. You need tight control over this ratio to get the best shape memory effect. Heat treatments and adding other elements like copper or hafnium can also change the nitinol transition temperature and improve performance.
Optimal NiTi compositions for shape memory performance are close to equal parts nickel and titanium.
Adding elements like Hf or Cu and using special heat treatments can boost transformation temperatures and fatigue resistance.
Microstructural features, such as precipitates, help stabilize the transition and improve long-term function.
You can see that the nickel-titanium ratio and the nitinol transition temperature work together. They let you design nitinol tubing with the exact properties you need, from the right austenite finish temperature to the best shape memory effect.
Applications of Shape Memory Effect

Tubing Deformation and Recovery
When you use nitinol tubing, you see how it bends and returns to its original shape. This happens because of the shape memory effect. You can deform nitinol tubing by up to 4.16% strain, and it will recover its shape. In some cases, nitinol tubing can handle a superelastic strain of about 7%. If you bend the tubing too much, above 8% strain, it may not return fully. Most practical applications keep the strain lower to ensure reliable recovery.
Researchers have tested nitinol tubing using methods like Differential Scanning Calorimetry and Bend Free Recovery. These tests show that nitinol tubing keeps its shape memory and superelastic properties even after many cycles. For example, nitinol tubing can last up to 10 million cycles at strain amplitudes between 0.5% and 2.9%. This makes it perfect for biomedical devices that need to work for a long time without failing.
Alternating Strain (%) | Median Fatigue Life (cycles) ± Std Dev (Air, 72 RPM) |
|---|---|
2.0 | 1,020 ± 91 |
1.2 | 6,828 ± 578 |
0.8 | 48,678 ± 494,567 |
Tip: You should keep the strain within safe limits to get the best performance from nitinol tubing in your devices.
Practical Uses
You find nitinol in many practical applications because of its unique properties. In the biomedical field, nitinol tubing is used in stents, implants, and artificial heart valves. These biomedical devices take advantage of nitinol’s ability to recover shape and resist fatigue. Clinical studies show that nitinol stents help patients recover faster and lower the risk of complications compared to traditional materials.
Nitinol also plays a big role in aerospace and industrial applications. You see it in actuators, sensors, and morphing wings. Its lightweight and corrosion resistance make it ideal for harsh environments. In cooling and heating systems, nitinol wires absorb or release heat when deformed, creating temperature changes of about 20 °C. This effect is now used in automotive air conditioning and residential climate control.
Metric | Result for 0.05mm Wall Thickness Nitinol Tubing |
|---|---|
Tensile Strength | 500–900 MPa |
Local Strains | Up to 6% |
Cycles to Failure | Up to 10 million cycles |
Strain Amplitudes | 0.5%–2.9% |
Cold Work Percentage | 20%–30% |
You see the applications of nitinol in both medical and industrial settings. Its shape memory effect supports practical applications that demand reliability and long-term performance. In biomedical devices, nitinol tubing ensures safe and effective treatments. In industry, it reduces vibration and improves sensor accuracy. The practical applications of nitinol continue to grow as new technologies emerge.
You have seen how nitinol tubing uses atomic-level phase changes to keep its memory. The nickel-titanium ratio and nitinol transition temperature work together to control when the tubing returns to its original shape. By setting the nitinol transition temperature near body temperature, you ensure safe and reliable medical applications. Nitinol’s memory and superelasticity make it valuable in many fields.
Looking ahead, you will find new directions for shape memory materials:
Shape memory composites with self-healing and self-sensing features
Advanced nitinol alloys for better strength and biocompatibility
3D printing and nanotechnology for custom, high-performance applications
FAQ
What makes nitinol different from other metals?
You see nitinol act in ways that most metals cannot. Its atoms shift together without breaking apart. This lets nitinol return to its original shape after bending. You find this unique property in many important applications.
How does temperature affect nitinol tubing?
When you heat or cool nitinol tubing, you trigger a phase change. The tubing shifts between two atomic structures. This change lets the tubing remember and recover its shape. You can use this effect in many applications.
Can nitinol tubing be used in biomedical devices?
Yes, you often find nitinol tubing in biomedical devices. Its shape memory and superelasticity help doctors create safe and reliable tools. You see nitinol used in stents and other medical applications.
Why is nitinol popular in industrial applications?
You choose nitinol for industrial applications because it resists fatigue and returns to its shape quickly. Its memory effect works well in sensors, actuators, and other devices. You get long-lasting performance from nitinol in many fields.
See Also
Understanding Nitinol's Unique Shape Memory And Elastic Properties
The Process Of Producing Nitinol Tubing For Healthcare Use
Nitinol Tubing's Impact On Progress In Medical Innovations
Transforming Medical Devices Through Advanced Nitinol Tubing

