Exploring Nitinol Tubing and Its Role in 2025 Healthcare
Nitinol tubing, a revolutionary material in modern healthcare, combines superelasticity and shape memory to create highly adaptable medical devices. This unique alloy of nickel and titanium responds to temperature changes, allowing it to return to a pre-defined shape, which enhances its performance in dynamic environments. Its biocompatibility ensures safety while its durability supports long-term use in critical procedures.
In 2025, nitinol plays a pivotal role in advancing medical technology. The global market for nitinol tubing, valued at USD 1.2 billion in 2024, is projected to grow significantly. By 2033, it is expected to reach USD 2.5 billion, driven by a compound annual growth rate of 9.5% from 2026. This growth reflects its increasing adoption in life-saving applications, including cardiovascular implants and neurovascular devices.
Key Takeaways
Nitinol tubing is stretchy and remembers shapes, perfect for medical tools.
The growing nitinol market shows its value in saving lives.
Nitinol is safe and strong, great for long-term medical use.
New ways to make nitinol improve its quality and accuracy.
Nitinol helps patients in heart surgeries and robotic procedures.
Unique Properties of Nitinol Tubing
Superelasticity in Medical Devices
Superelasticity is one of the most remarkable properties of nitinol tubing, making it indispensable in modern medical applications. This property allows nitinol to undergo significant deformation and return to its original shape without permanent damage. Superelastic nitinol tubing is particularly valuable in dynamic environments, such as the human body, where flexibility and adaptability are critical.
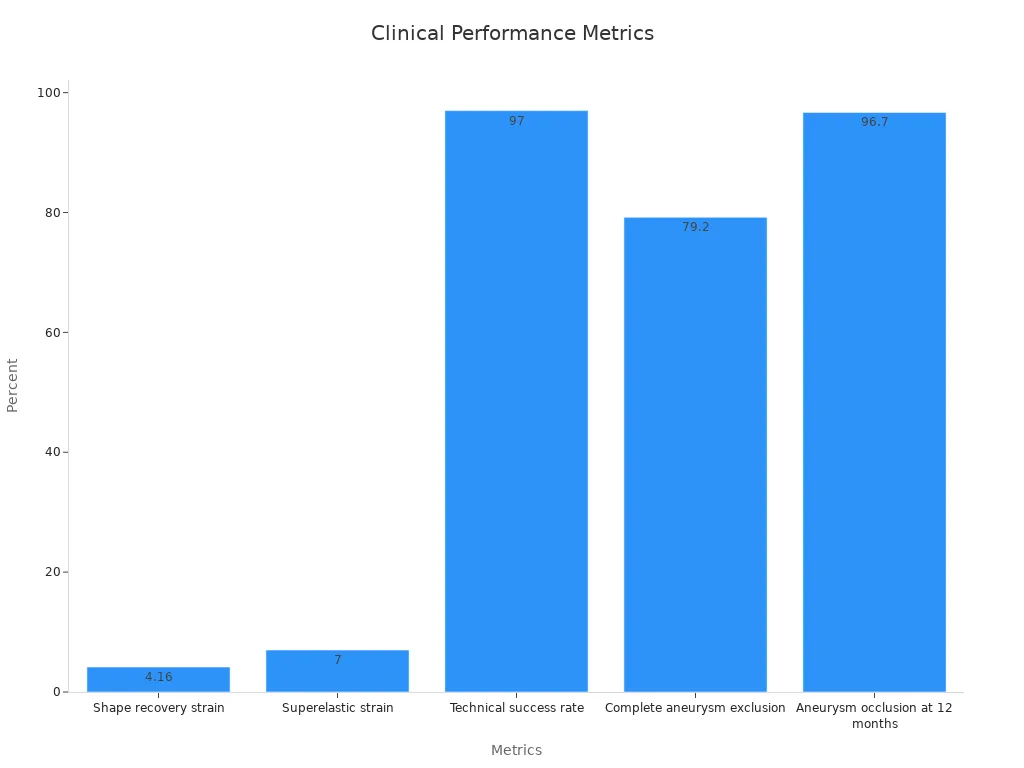
Clinical trials have demonstrated the exceptional performance of superelastic nitinol tubing. For instance, it exhibits a shape recovery strain of 4.16% and a superelastic strain of 7%. These metrics far exceed those of conventional materials like stainless steel, which can only tolerate 1% strain. Additionally, the technical success rate of nitinol-based devices in clinical settings has reached 97%, with 96.7% aneurysm occlusion at 12 months.
Metric | Result |
|---|---|
Shape recovery strain | 4.16% |
Superelastic strain | 7% |
Technical success rate | 97% (70 of 72 patients) |
Complete aneurysm exclusion | 79.2% (57 of 72 patients) |
Aneurysm occlusion at 12 months | 96.7% (29 of 30 patients) |
Experimental methods like Differential Scanning Calorimetry (DSC) and Bend Free Recovery (BFR) tests further validate the superelasticity of nitinol tubing. DSC measures transformation temperatures, while BFR assesses shape recovery after deformation, highlighting the material's ability to adapt to varying conditions.
Experimental Method | Description | Significance |
|---|---|---|
Differential Scanning Calorimetry (DSC) | Measures transformation temperatures of nitinol | Essential for understanding phase changes between austenite and martensite |
Bend Free Recovery (BFR) Test | Assesses shape recovery after deformation | Demonstrates superelasticity and characterizes Active Austenite Finish (Active Af) temperature |
Shape Memory and Its Healthcare Benefits
Nitinol tubing's shape memory property enables it to return to a pre-defined shape when exposed to specific temperatures. This unique characteristic, inherent to shape memory alloys like nitinol, has revolutionized healthcare by enhancing the reliability and effectiveness of medical devices.
Devices such as self-expanding stents leverage this property to conform to the body's anatomy, ensuring precise deployment and expansion. Nitinol's shape memory also allows devices to adapt to body movements, reducing complication risks and improving fixation device performance. Clinical tests, including cyclic life testing, have validated the reliability of this property, emphasizing its role in complex surgical procedures.
Nitinol's shape memory enables devices to adapt to body movements, reducing complication risks and enhancing fixation device effectiveness.
Superelastic nitinol tubing can undergo elastic deformation up to 10% strain, significantly outperforming conventional materials like stainless steel, which only allows 1% strain.
Devices like self-expanding stents utilize nitinol's shape memory to conform to the body's anatomy, allowing for precise expansion after deployment.
Biocompatibility and Durability
The biocompatibility and durability of nitinol tubing make it a preferred choice for long-term medical applications. Its biocompatibility minimizes adverse reactions, while its corrosion resistance prevents harmful ion release, ensuring safety. The material's surface characteristics, including titanium oxides, further enhance its compatibility with biological systems.
Long-term studies have highlighted nitinol's durability, showing that it can endure high-cycle fatigue life, which is essential for vascular stents and other critical devices. Processing methods like TM-1 and low-temperature aging improve fatigue resistance, ensuring reliability in demanding applications.
Nitinol tubing exhibits excellent biocompatibility, minimizing adverse reactions in the human body.
Its corrosion resistance prevents harmful ion release, enhancing safety.
The material's surface characteristics, including titanium oxides, improve compatibility with biological systems.
Nitinol's durability is critical, with studies indicating it can endure high-cycle fatigue life, essential for vascular stents.
Laser-cut nitinol devices can withstand fatigue strain limits of 0.4–0.8% under clinically relevant conditions.
The combination of biocompatibility and corrosion resistance ensures that nitinol tubing remains a reliable material for medical devices. Its ability to maintain performance under extreme conditions underscores its importance in advancing healthcare technologies.
Applications of Medical Grade Nitinol Tubing in 2025

Cardiovascular Implants and Stents
Cardiovascular implants and stents represent one of the most transformative applications of nitinol tubing in 2025. Nitinol's superelasticity and shape memory properties enable stents to adapt to the dynamic environment of blood vessels. These implants expand precisely within arteries, restoring blood flow and reducing the risk of restenosis.
The biocompatibility of nitinol tubing ensures safety during long-term use, minimizing adverse reactions. Its corrosion resistance prevents the release of harmful ions, enhancing reliability in critical procedures. Additionally, nitinol's flexibility allows stents to conform to the natural movements of the cardiovascular system, improving patient outcomes.
Medical grade nitinol tubing has also impacted implant design and performance. Customization for orthopedic implants has become more advanced, allowing for tailored solutions that meet individual patient needs. This technological advancement has made minimally invasive surgeries more effective, reducing recovery times and improving overall safety and functionality.
Therapeutic Catheters and Guidewires
Therapeutic catheters and guidewires benefit significantly from the unique properties of nitinol tubing. The material's strength and flexibility allow these devices to navigate complex anatomical pathways with ease. Nitinol tubing ensures that catheters and guidewires maintain their shape during insertion, reducing the risk of damage and enhancing reliability.
In minimally invasive surgeries, nitinol-based catheters and guidewires play a crucial role. Their ability to adapt to the body's contours ensures precise delivery of therapeutic agents, improving treatment outcomes. The durability of nitinol tubing supports repeated use in demanding medical applications, making it a preferred choice for healthcare providers.
The customization of nitinol tubing for therapeutic devices has advanced significantly. Manufacturers now produce catheters and guidewires with enhanced biocompatibility, ensuring safety during prolonged procedures. These innovations have expanded the scope of technological applications, enabling more effective treatments for a wide range of conditions.
Neurovascular and Brain-Related Devices
Neurovascular and brain-related devices rely heavily on nitinol tubing for their functionality and safety. Nitinol's superelasticity allows devices like stents and catheters to maintain their shape after bending, enabling them to navigate intricate blood vessels without sustaining damage. This characteristic enhances the safety and effectiveness of neurovascular procedures.
Clinical trials have demonstrated the reliability of nitinol tubing in these applications:
Coiling procedures achieved a success rate of 71.4%.
Flow diversion stenting reached a success rate of 77.9%.
Choosing safe neurovascular devices is very important. Groups like the FDA and EMA check these devices carefully. They make companies test devices in clinical trials before approval. These tests ensure the devices work well and are safe to use.
Medical grade nitinol tubing has revolutionized the design of neurovascular devices. Its biocompatibility minimizes adverse reactions, while its strength ensures durability during complex procedures. These advancements have made nitinol tubing indispensable in the development of brain-related implants and therapeutic tools.
Surgical tools and robotic-assisted procedures
Nitinol tubing has transformed surgical tools and robotic-assisted procedures by enhancing precision, reliability, and safety. Its unique properties, including superelasticity and biocompatibility, make it an ideal material for advanced medical applications. Surgical tools made from nitinol tubing adapt to complex anatomical structures, ensuring accurate performance during intricate procedures.
Robotic-assisted surgeries benefit significantly from nitinol's flexibility and durability. Instruments equipped with nitinol tubing can bend and return to their original shape without damage, allowing surgeons to navigate challenging environments with ease. This adaptability reduces the risk of complications and improves patient outcomes.
The integration of nitinol tubing into robotic systems has led to remarkable advancements in orthopedic implants and minimally invasive surgeries. These systems utilize nitinol-based tools to perform precise movements, ensuring optimal placement of implants and reducing recovery times. The material's corrosion resistance and fatigue life further enhance the reliability of these tools, making them indispensable in modern healthcare.
Clinical studies highlight the superior performance of nitinol tubing in surgical applications. For instance, the DAWN Trial demonstrated that nitinol-based tools achieved 49% functional independence at 90 days, compared to 13% with standard care. Similarly, the PARTNER 3 Trial reported a 1.0% mortality rate at one year for nitinol-assisted procedures, significantly lower than the 2.5% rate for open-heart surgery.
Study Name | Nitinol Outcome | Standard Care Outcome |
|---|---|---|
DAWN Trial | 49% functional independence at 90 days | 13% functional independence |
PARTNER 3 Trial | 1.0% mortality rate at one year | 2.5% mortality rate for open-heart surgery |
Robotic-assisted procedures using nitinol tubing have set new benchmarks for precision and safety. These innovations continue to redefine the boundaries of medical applications, offering hope for improved patient care and outcomes.
The versatility of nitinol tubing extends to surgical tools used in orthopedic implants. These tools leverage nitinol's shape memory to conform to the body's anatomy, ensuring accurate placement and reducing the risk of misalignment. The material's biocompatibility minimizes adverse reactions, while its durability supports long-term use in demanding medical environments.
Manufacturers have refined the design of nitinol-based surgical tools to meet the evolving needs of healthcare providers. Innovations in precision engineering have enabled the production of tools with enhanced reliability and performance. These advancements have expanded the scope of nitinol tubing applications, solidifying its role in robotic-assisted procedures and surgical tools.
Advancements in Nitinol Tubing Manufacturing
Innovations in Precision Engineering
Recent advancements in nitinol tubing manufacturing have revolutionized precision engineering. Manufacturers now achieve tight dimensional tolerances as low as ±0.0005 inches, ensuring consistent performance across various medical applications. This level of precision enhances the reliability of nitinol tubing in critical procedures, such as cardiovascular and neurovascular interventions.
Advanced polishing techniques have also improved surface quality, reducing surface roughness. This enhancement increases fatigue resistance and biocompatibility, making nitinol tubing safer and more durable for long-term use. Engineers have further refined customization capabilities, tailoring tubing to specific requirements like wall thickness, diameter, and mechanical properties. These innovations allow for the creation of custom nitinol tubing that meets the unique demands of modern medical devices.
Key Features | Description |
|---|---|
Tight Dimensional Tolerances | Achieved tolerances as low as ±0.0005 inches, ensuring consistent performance across applications. |
Improved Surface Quality | Advanced polishing techniques reduced surface roughness, enhancing fatigue resistance and biocompatibility. |
Customizability | Engineers could tailor tubing to specific requirements, including wall thickness, diameter, and mechanical properties. |
Enhanced Material Quality and Consistency
Enhanced material quality and consistency have become a cornerstone of nitinol tubing manufacturing. Non-destructive testing methods, such as ultrasonic and eddy current testing, ensure the integrity of the material without causing damage. These techniques detect flaws early in the production process, maintaining high standards of quality.
Dimensional inspections verify that nitinol tubing meets precise specifications for outer diameter, wall thickness, and concentricity. Mechanical testing evaluates the tubing's strength, elasticity, and fatigue resistance, ensuring it can withstand the demands of medical applications. These rigorous quality control measures align with regulatory compliance for medical grade nitinol tubing, ensuring safety and reliability.
Quality Control Measure | Description |
|---|---|
Non-Destructive Testing | Ensures quality without damaging the material, using techniques like ultrasonic and eddy current testing to detect flaws. |
Dimensional Inspections | Verifies that tubing meets precise specifications for outer diameter, wall thickness, and concentricity. |
Mechanical Testing | Evaluates strength, elasticity, and fatigue resistance through tensile and bend tests. |
Global Supply Chain Improvements
Global supply chain improvements have played a vital role in advancing nitinol tubing technology. Manufacturers have streamlined production processes, reducing lead times and ensuring timely delivery of medical-grade nitinol tubing. These improvements have supported the growing demand for nitinol in healthcare, particularly in minimally invasive surgeries and robotic-assisted procedures.
Collaborations between manufacturers and suppliers have enhanced the availability of high-quality raw materials. This has minimized variability in nitinol's composition, ensuring consistent performance across applications. Additionally, advancements in logistics technology have optimized the transportation of nitinol tubing, reducing the risk of damage during transit.
The global supply chain for nitinol tubing has become more resilient, enabling healthcare providers to access reliable materials for life-saving medical devices.
These advancements in nitinol tubing manufacturing have set new benchmarks for quality, precision, and efficiency, solidifying nitinol's role as a transformative material in modern healthcare.
Nitinol tubing has redefined modern healthcare with its superelasticity, shape memory, and biocompatibility. These properties have enabled groundbreaking applications, from cardiovascular implants to robotic-assisted surgeries. A recent study on nitinol stents for cerebral aneurysms showed an 89% occlusion rate within 18 months, highlighting its role in improving surgical outcomes. Long-term evaluations also confirm its structural integrity, ensuring reliability in demanding medical procedures. As innovations in nitinol manufacturing continue, its transformative impact on healthcare in 2025 underscores the need for sustained research and development to unlock its full potential.
FAQ
What is nitinol tubing used for in healthcare?
Nitinol tubing is used to create medical devices like stents, catheters, and surgical tools. Its unique properties, such as superelasticity and shape memory, make it ideal for minimally invasive procedures and long-term implants.
How does nitinol improve medical device performance?
Nitinol enhances device performance by providing flexibility, durability, and biocompatibility. These features allow devices to adapt to the human body, ensuring safety and reliability during critical procedures.
Why is nitinol tubing preferred for cardiovascular applications?
Nitinol tubing is preferred because it can expand and conform to blood vessels. This adaptability improves the effectiveness of cardiovascular implants, such as stents, while reducing the risk of complications.
What makes nitinol biocompatible?
Nitinol’s biocompatibility comes from its titanium oxide surface layer, which minimizes adverse reactions. This property ensures safety for long-term use in medical applications.
How has nitinol tubing manufacturing advanced?
Manufacturing advancements include tighter dimensional tolerances, improved surface quality, and enhanced material consistency. These innovations ensure nitinol tubing meets the high standards required for modern healthcare.
See Also
Nitinol Tubing's Impact on Healthcare Device Innovations
Nitinol Tubing's Contribution to Modern Medical Technology
Nitinol Tubes: Pioneering the Next Generation of Medical Devices
Transforming Medical Devices Through Nitinol Tubing Innovations
The Essential Role of Nitinol Tubing in Medical Advancements

