Step-by-Step Guide to Using Nitinol Tubing for Thrombectomy Stents
Nitinol tubing for thrombectomy stent manufacturing plays a pivotal role in advancing neurovascular care. Nitinol offers exceptional flexibility, superelasticity, and shape memory, making it ideal for stents designed for mechanical thrombectomy. These properties enable reliable removal of clots and improved vessel patency. The evolution from traditional materials to nitinol tubing for thrombectomy stent has led to customizable nitinol stents with higher success rates and fewer complications. The transformative impact on neurovascular care appears clear when examining outcomes such as a 100% technical success rate and over 90% primary patency at six months for stents used in treatment.
Metric
Value
Context
Technical success rate
100%
Hemobahn nitinol stent study
Primary patency (6 months)
90%
Femoral artery stents
Limb salvage
98% (stent)
Compared to 89% (bypass)
Nitinol enables safe, effective thrombectomy and supports ongoing improvement in treatment and removal of clots for better patient outcomes.
Key Takeaways
Nitinol tubing offers unique flexibility and shape memory, making it ideal for thrombectomy stents that safely remove clots and improve blood flow.
Advanced manufacturing steps like gun drilling, laser cutting, and heat treatment ensure nitinol stents are precise, durable, and biocompatible.
Customizable nitinol stents fit patient-specific vessel shapes, enhancing treatment success and reducing complications.
Clinical studies show nitinol stents achieve high clot removal rates, maintain vessel openness, and support long-term patient health.
Nitinol stents combine strength and safety, making them a trusted choice for modern neurovascular care and future medical innovations.
Nitinol Tubing for Thrombectomy Stent Manufacturing
Nitinol Properties
Nitinol stands out as a unique material in the field of stents due to its remarkable mechanical and physical characteristics. As a member of the shape memory alloys family, nitinol exhibits both superelasticity and shape memory. These features allow stents to recover their original shape after deformation and to adapt to the dynamic environment inside blood vessels. Superelastic nitinol alloys can undergo significant strain and return to their initial form, which is essential for devices that must navigate tortuous vascular pathways.
Laboratory tests confirm the exceptional performance of nitinol.
In situ neutron diffraction experiments reveal how nitinol’s Young’s modulus changes with phase, ranging from 20–50 GPa in martensite to 40–90 GPa in austenite.
Compression and tension tests show transformation strains between 3.5% and 4.3%, with transformation stresses from 200 to 500 MPa.
Heat treatment at 500°C can enhance superelastic recovery strain up to 6%.
Tensile testing demonstrates that nitinol tubing for thrombectomy stent manufacturing can withstand strains up to 5.11%, matching traditional nitinol parts.
Why Nitinol for Mechanical Thrombectomy
Nitinol tubing for thrombectomy stent manufacturing has become the industry standard because of its ability to combine flexibility, strength, and biocompatibility. The global medical grade hypotube market, which includes nitinol tubing for thrombectomy stent, reached $3.2 billion in 2023 and is projected to grow to $5.8 billion by 2032, with a 6.5% CAGR. Nitinol holds about 40% of the endovascular material market, and thrombectomy stents account for roughly 10% of the stent segment.
Clinical studies highlight the superiority of nitinol in mechanical thrombectomy. Devices like EmboTrap and Trevo, made from nitinol, achieve better neurological outcomes and lower rates of hemorrhage and mortality compared to older stents. The TREVO 2 trial showed that nitinol-based retrievers outperform non-nitinol devices in clot removal and long-term patient recovery. Nitinol’s superelasticity and shape memory enable stents to conform to complex vessel shapes, optimize clot engagement, and minimize vessel injury. These advantages explain why applications of nitinol stents continue to expand in neurovascular and cardiovascular care.
Nitinol tubing for thrombectomy stent manufacturing supports the development of customizable, high-performance stents that improve patient outcomes and set new standards for safety and efficacy in thrombectomy.
Manufacturing Process
Gun Drilling and Tubing Formation
The manufacturing process for nitinol tubing begins with a solid nitinol rod. Engineers use gun drilling to create a precise hollow tube. This step requires high accuracy because the tubing must meet strict dimensional standards for use in stents. Gun drilling achieves over 99% concentricity, which ensures uniform mechanical properties throughout the tubing. Dimensional accuracy testing confirms that wall thickness tolerance stays within 0.01 mm, and surface roughness remains at or below 0.1 μm. These tight tolerances help prevent device failure and support the long-term durability of nitinol stents.
Tip: Quality control tools such as laser micrometers and ultrasonic thickness gauges help maintain these standards and detect any deviations early in the process.
Process Efficiency Metric | Description and Impact |
|---|---|
Gun Drilling Precision | Over 99% concentricity for uniform properties |
Dimensional Accuracy | Wall thickness tolerance within 0.01 mm; surface roughness Ra ≤ 0.1 μm |
Mechanical Stability | 20 cycles of 6% strain recovery testing confirm tubing can withstand repeated stress |
Microstructure Control | Porosity and inclusions controlled within 5.4 μm and 0.5% area ratio for improved durability |
Laser Cutting and Microfabrication
After forming the tubing, manufacturers use laser cutting to define the stent geometry. This step shapes the nitinol tubing into intricate patterns that allow the stents to expand and contract as needed. Femtosecond lasers can achieve cut widths as small as 30 μm, while Nd:YAG lasers produce cuts between 50 and 100 μm. Shorter laser pulses reduce thermal damage and improve the fidelity of the cuts. These advanced microfabrication techniques enable the creation of complex stent designs that maximize flexibility and minimize trauma to blood vessels.
Laser cutting also plays a critical role in removing burrs and sharp edges. This step is essential for patient safety and for optimizing the superelasticity and shape memory of nitinol stents. Quality control data show that microstructural characteristics, such as grain size and inclusion density, are closely monitored using optical and scanning electron microscopy. Automated image analysis detects non-metallic inclusions, which can act as crack nucleation sites and reduce the long-term durability of the stents.
Heat and Chemical Treatment
Heat and chemical treatments further enhance the properties of nitinol tubing. Heat treatment stabilizes the mechanical properties and superelasticity of the material. Manufacturers typically use ageing temperatures of 400°C, 500°C, or 600°C, with dwell times ranging from 30 to 90 minutes. These parameters influence the recrystallization and phase composition of nitinol, which directly affect the performance of the stents.
Chemical etching improves the surface morphology and topography of nitinol tubing. Common chemical mixtures include HCl/H2SO4, H2SO4/H2O2, and NH4OH/H2O2. For example, a two-hour treatment with NH4OH/H2O2 creates deep cracks and plate-like structures that promote cell growth and enhance biocompatibility. The formation of a thick titanium oxide layer during etching increases corrosion resistance and supports the long-term durability of nitinol stents. Two-stage chemical etching, such as HCl/HF/H3PO4 followed by HNO3/HCl, significantly improves cell adhesion and proliferation.
Ageing temperatures: 400°C, 500°C, 600°C
Dwell times: 30, 60, 90 minutes
Chemical etching: HCl/H2SO4, H2SO4/H2O2, NH4OH/H2O2
Surface effects: Hydrophilic species, thick titanium oxide layer
Surface Finishing
Surface finishing is the final step in the manufacturing process. This stage focuses on achieving a smooth, defect-free surface that resists corrosion and supports biocompatibility. Electropolishing and chemical etching remove surface irregularities and improve the fatigue life of nitinol stents. Surface roughness is maintained at or below 0.1 μm, and wall thickness tolerances remain within 0.01 mm. These measures help prevent corrosion and rust, which are critical for the safety and effectiveness of the stents.
Corrosion resistance testing shows that electropolished nitinol tubing exhibits no breakdown potential up to 1000 mV, while oxidized tubing breaks down at much lower voltages. This difference highlights the importance of high-quality surface finishing for medical applications. Comparative testing of post-processing methods reveals that a hybrid additive-subtractive approach achieves superior dimensional precision, while chemical etching combined with electropolishing effectively smooths both external and internal surfaces. Recovery percentages of shape-memory behavior above 75% confirm that these finishing steps maintain the functional quality of nitinol stents.
Note: Adherence to ASTM, ISO, and FDA standards ensures that every nitinol stent meets the highest requirements for safety, reliability, and effectiveness.
Customizable Nitinol Stents
Design Flexibility
Customizable nitinol stents offer unmatched design flexibility for modern medical needs. Manufacturers can adjust the size, shape, and geometry of each stent to fit the unique anatomy of every patient. Nitinol tubing supports precise control over dimensions, with tubing diameters as small as 0.3 mm and wall thicknesses ranging from 0.1 mm to 15 mm. This tight control ensures that stents perform reliably in complex neurovascular and cardiovascular procedures.
Engineers use advanced microfabrication techniques, such as laser cutting and electropolishing, to create intricate stent patterns. These methods allow for open-cell or closed-cell designs, each providing different balances of flexibility and strength. Adjustments to wire diameter, braiding angle, and stent diameter help tailor mechanical properties like reaction force and bending stiffness. For example, a stent with a 15 mm diameter, 60° braiding angle, and 0.09 mm wire diameter achieves excellent flexibility and minimal bending stiffness. This high degree of customization supports safe and effective deployment in challenging vessel anatomies.
The ability to create custom nitinol stents with patient-specific features leads to better clinical outcomes and reduces the risk of complications.
Evidence Aspect | Supporting Details |
|---|---|
Nitinol Properties | Shape memory and superelasticity enable stents to conform to complex vessel anatomies, providing uniform radial force and improved clinical outcomes. |
Technological Advancements | Laser cutting and electropolishing improve precision, biocompatibility, and durability of nitinol stents, enabling sophisticated, thin-walled designs. |
Customization Trends | Emphasis on patient-specific implants, expanded use in robotic-assisted surgeries, advancements in minimally invasive device design, integration of intelligent sensor technologies |
Hybrid Materials and Advanced Features
Customizable nitinol stents now integrate hybrid materials and advanced features to further enhance performance. Manufacturers combine nitinol with shape memory polymer foams, radiopaque markers, or drug-eluting coatings. These additions improve visibility during procedures, support targeted drug delivery, and increase biocompatibility. Hybrid designs also allow for the creation of stents that resist kinking and maintain vessel patency over time.
The applications of nitinol stents continue to expand. In vascular, neurovascular, and even non-vascular fields, custom nitinol stents adapt to a wide range of clinical needs. Market analysis shows that stents hold the largest revenue share among nitinol medical devices, with a projected market size of $31.6 billion by 2029 and a CAGR of 10.6%. The growing aging population and rising rates of cardiovascular disease drive demand for advanced, customizable nitinol stents. Hospitals and clinics increasingly choose these devices for their durability, fatigue resistance, and ability to withstand millions of cycles without failure.
Custom nitinol stents support minimally invasive procedures.
Advanced surface coatings and microfabrication techniques extend device lifespan.
Healthcare providers value the adaptability of nitinol for complex anatomies.
The applications of nitinol stents in robot-assisted and image-guided procedures highlight their versatility. As technology advances, customizable nitinol stents will continue to set new standards for safety, precision, and patient-specific care.
Clinical Benefits of Nitinol Tubing for Thrombectomy Stent
Self-Expansion and Vessel Patency
Nitinol stents deliver reliable self-expansion, which helps maintain vessel patency after the removal of clots. The superelastic properties of nitinol allow stents to adapt to vessel walls, supporting the effective removal of clots and reducing the risk of restenosis. This flexibility leads to improved performance and reduces complications during neurovascular care. Clinical studies show that nitinol stents achieve high rates of vessel healing and low rates of complications. For example:
Nitinol stents used in cerebral aneurysm treatment achieved complete or near-complete occlusion in up to 89% of cases within 18 months.
Flow-diverting nitinol stents reached a 76% complete occlusion rate in complex aneurysms, with low morbidity (5%) and mortality (4%) rates.
Devices like PulseRider reported occlusion rates around 90% and a 5% complication rate.
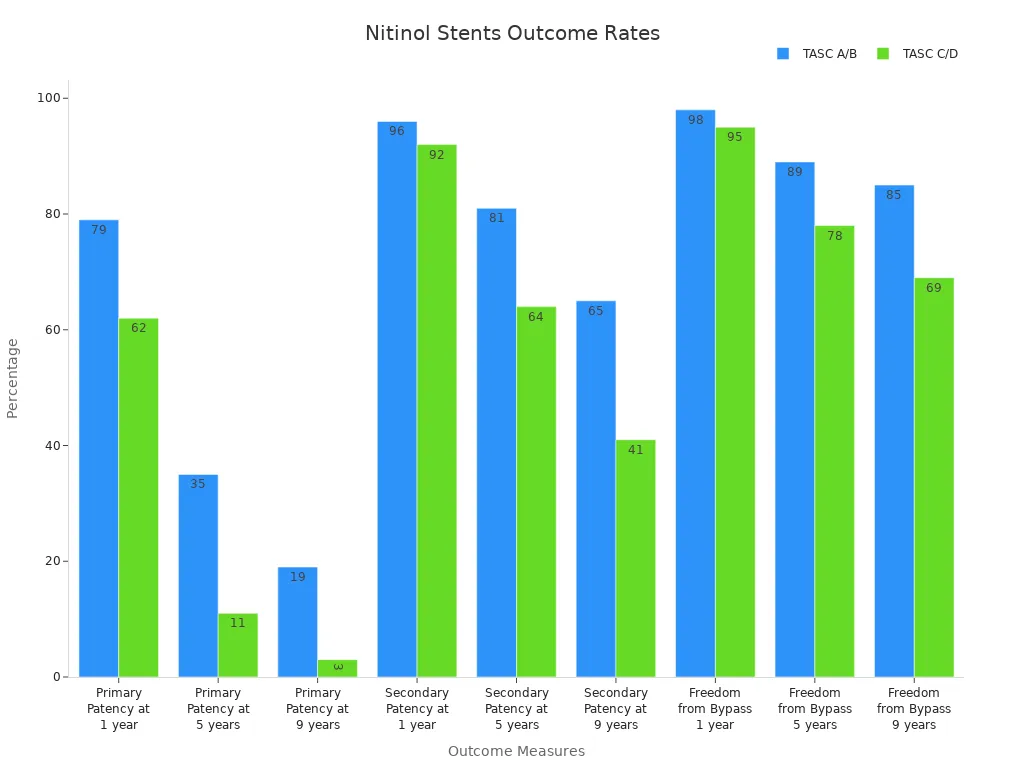
These outcomes highlight the real-world uses and benefits of nitinol tubing in neurovascular care. The following table summarizes long-term patency and bypass freedom rates for nitinol stents:
Outcome Measure | TASC A/B Lesions (%) | TASC C/D Lesions (%) |
|---|---|---|
Primary Patency at 1 year | 79 | 62 |
Primary Patency at 5 years | 35 | 11 |
Primary Patency at 9 years | 19 | 3 |
Secondary Patency at 1 year | 96 | 92 |
Secondary Patency at 5 years | 81 | 64 |
Secondary Patency at 9 years | 65 | 41 |
Freedom from Bypass at 1 year | 98 | 95 |
Freedom from Bypass at 5 years | 89 | 78 |
Freedom from Bypass at 9 years | 85 | 69 |
Below Knee Amputations | 5 (total observed) | N/A |
Navigation and Clot Retrieval
Nitinol tubing enables stents to navigate complex vascular pathways with ease. The material’s flexibility and superelasticity allow for enhanced precision in neurovascular procedures. Stents made from nitinol demonstrate high clot retrieval success rates, supporting the removal of clots in challenging cases. In controlled models, Solitaire FR and new nitinol stent designs achieved clot retrieval rates above 95%. These stents also require low recapture force, which simplifies repositioning and removal. This efficiency leads to shorter procedure times and less exposure to anesthesia and radiation, improving treatment outcomes for patients.
Durability and Biocompatibility
Durability remains a key advantage of nitinol in thrombectomy stent performance. Nitinol stents withstand millions of fatigue cycles, even in demanding environments near the heart. Devices endure up to 400 million cycles, far surpassing other metals. Nitinol can tolerate cyclic strain amplitudes between 4% and 12%, while other alloys fail at 1% or less. This long-term durability ensures that stents maintain vessel patency and reduce the risk of complications over time. Material processing, such as low-temperature aging and surface treatments, further enhances fatigue resistance. These features support durability and reduced risk of complications, making nitinol the preferred choice for neurovascular care and the removal of clots. Success stories in clot removal and improved performance reduces complications, leading to better treatment outcomes and patient quality of life.
Nitinol tubing for thrombectomy stent manufacturing has transformed neurovascular care by combining shape memory, superelasticity, and biocompatibility. Stents made from nitinol achieve high recanalization rates, low complication rates, and strong patient outcomes. Manufacturing steps like heat treatment and surface finishing enhance durability and safety. Devices such as EMBOTRAP and Solitaire FR demonstrate rapid clot removal and lasting vessel patency. Future innovations in nitinol will continue to improve outcomes and expand possibilities in neurovascular care.
FAQ
What makes nitinol tubing ideal for thrombectomy stents?
Nitinol tubing provides superelasticity and shape memory. These properties help stents expand and fit blood vessels. Nitinol also resists corrosion and fatigue, which increases device safety and lifespan.
How does laser cutting improve stent performance?
Laser cutting creates precise patterns in nitinol tubing. This process allows engineers to design stents with optimal flexibility and strength. Smooth edges from laser cutting also reduce the risk of vessel injury.
Can nitinol stents be customized for each patient?
Yes. Manufacturers adjust nitinol stent size, shape, and features to match patient anatomy. Customization improves fit and performance, leading to better clinical outcomes.
Are nitinol stents safe for long-term use?
Clinical studies show nitinol stents remain durable and biocompatible over many years. Surface treatments and quality control further reduce risks. Most patients experience improved vessel patency and fewer complications.
See Also
Complete Process for Creating Nitinol Microtubing in Neurovascular Use
How To Choose The Best Nitinol Tubing For Your Needs
Understanding The Production Of Nitinol Tubing For Medical Use
The Importance Of Nitinol Tubing In Minimally Invasive Surgery
Discovering Various Uses Of Nitinol Tubing In Medical Devices

