How Nitinol Tubing is Used in CE-Marked Stent Retrievers
Nitinol tubing for ce-marked stent retrievers gives you a powerful tool for treating blocked arteries. The unique properties of nitinol, like shape memory and superelasticity, allow these medical devices to self-expand and adapt to the vessel. Nitinol tubing supports effective clot removal by ensuring the device can conform to complex anatomy. Clinical trials show that nitinol-based devices achieve high performance and safety in acute stroke treatment. You benefit from nitinol tubing’s proven reliability and the way it helps meet strict standards for medical devices. These properties make nitinol tubing for ce-marked stent retrievers essential in many applications, driving innovation for modern devices.
Key Takeaways
Nitinol tubing offers unique shape-memory and superelasticity that help stent retrievers expand and adapt inside blood vessels for effective clot removal.
The manufacturing process of nitinol tubing includes precise drawing, laser cutting, heat setting, and surface finishing to ensure high quality, safety, and durability.
Nitinol tubing’s biocompatibility and corrosion resistance make it safe for long-term use in the body, reducing complications and improving patient outcomes.
CE-marked stent retrievers using nitinol tubing meet strict international standards, ensuring reliable performance and regulatory compliance.
Using nitinol tubing in stent retrievers improves flexibility, durability, and navigation through complex vessels, leading to better clinical success in stroke treatment.
Nitinol Tubing for CE-Marked Stent Retrievers
Nitinol Properties
You encounter nitinol as a unique alloy made from nearly equal parts nickel and titanium. This combination gives nitinol tubing for ce-marked stent retrievers a set of properties that make it ideal for medical devices. The alloy’s density ranges from 6.45 to 6.75 g/cm³, and its composition can be adjusted to fine-tune transformation temperatures and mechanical strength. Even small changes in the nickel-to-titanium ratio can affect how the material behaves inside the body.
Material Property | Description |
|---|---|
Density | 6.45–6.75 g/cm³ |
Composition | ~50:50 nickel to titanium |
Shape-memory effect | Returns to original shape when heated above transformation temperature |
Superelasticity | Large elastic deformation at body temperature |
Mechanical strength | High flexibility and energy absorption |
Fatigue resistance | Withstands repeated loading cycles |
Biocompatibility | Safe for use in human tissue |
You see nitinol tubing used in stents because of its high fatigue resistance and ability to absorb energy. Technical studies show that nitinol’s mechanical behavior under cyclic loading supports its role as the core material in CE-marked stent retrievers. Manufacturers like EUROFLEX produce high-precision nitinol tubing with tight tolerances and advanced surface finishes, ensuring consistent performance and safety. These tubes meet ISO 9001 and ISO 13485 standards, which are essential for regulatory compliance and patient safety.
Shape Memory and Superelasticity
Shape-memory and superelasticity set nitinol apart from other metals. When you use nitinol tubing for ce-marked stent retrievers, you benefit from these two remarkable properties. Shape-memory means the material can return to its original form after deformation when exposed to a specific temperature. Superelasticity allows the tubing to stretch or compress and then recover its shape instantly at body temperature.
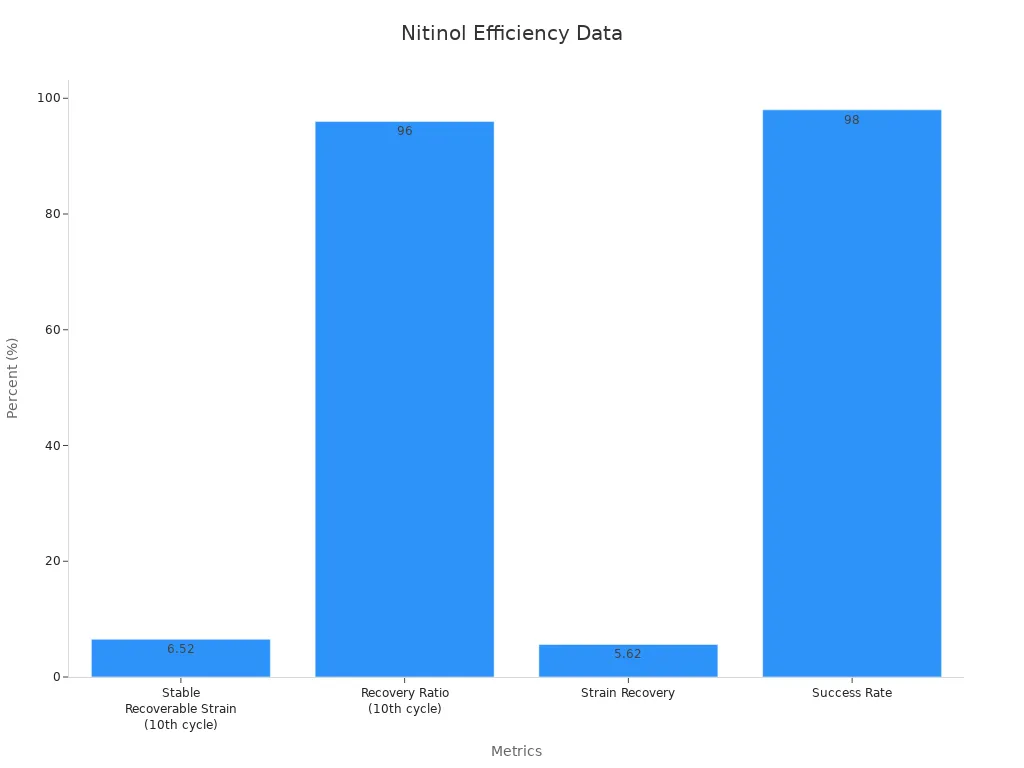
Experimental tension–torsion tests on thin-walled nitinol tubes confirm these behaviors. The tests show that nitinol undergoes reversible phase transformations between martensite and austenite structures. This transformation enables the tubing to recover from strains up to 6.3% without permanent deformation. Loading stresses range from 600 to 670 MPa, while unloading stresses fall between 254 and 288 MPa. These values ensure that stents made from superelastic nitinol tubing can expand reliably and adapt to vessel anatomy.
You find multiple stent retrievers, such as EmboTrap, Tigertriever, and the Phenox CRC, using nitinol tubing for their self-expanding function. Clinical data reveal a modified first-pass effect of 52.5% for self-expanding nitinol stent retrievers, demonstrating their effectiveness in restoring blood flow during mechanical thrombectomy. The superelasticity of nitinol tubing also provides kink resistance, which is crucial for navigating complex blood vessels.
Tip: Shape-memory and superelastic nitinol tubing allow stents to be compressed for delivery and then expand precisely at the treatment site, improving both performance and safety.
Biocompatibility
Biocompatibility is a critical property for any material used in medical devices. You need nitinol tubing that interacts safely with human tissue and blood. Nitinol’s biocompatibility comes from its stable oxide layer, which prevents harmful reactions and reduces the risk of corrosion. This property makes nitinol tubing for ce-marked stent retrievers suitable for long-term implantation.
Statistical studies confirm the safety and performance of nitinol tubing in medical applications. For example:
A study on superelastic nitinol tubing found high fatigue resistance, supporting its durability in stents.
Preclinical safety tests in animal models showed that nitinol devices caused only minor, acceptable tissue reactions.
Regulatory frameworks, such as FDA guidance and ISO 13485 certification, validate the safety and reliability of nitinol tubing.
You benefit from using nitinol tubing that meets ISO 13485 standards. This certification ensures that manufacturers follow strict quality management systems, reducing risks and improving product reliability. Certified nitinol tubing supports compliance with EU Medical Device Regulation (MDR) and FDA standards, making it easier for you to access global markets.
Note: Biocompatibility, combined with shape-memory and superelasticity, makes nitinol tubing the preferred choice for stents and other implantable devices. You gain confidence in both the safety and performance of your medical devices.
Manufacturing Process

The manufacturing of nitinol tubing for CE-marked stent retrievers involves several precise steps. You need to understand each stage to appreciate how these medical devices achieve their unique properties and reliability.
Tube Drawing
You start with high-purity nitinol alloy. The manufacturing process begins by drawing the alloy into seamless tubes. This step uses diamond dies and mandrels to control wall thickness and concentricity. You can achieve wall thickness tolerances as tight as ±0.0075 mm and surface roughness as low as Ra 0.1 μm. Cold working, which reduces the tube’s area by up to 30%, improves strength and flexibility. During manufacturing, you must perform rigorous testing, including ultrasonic and eddy current inspections, to detect internal and surface defects. Dimensional accuracy is checked with laser micrometers, ensuring the tubing meets strict standards for medical devices.
Performance Metric | Description |
|---|---|
Microstructure of Raw Materials | Tiny holes ≤ 5.4μm, unwanted particles ≤ 0.5% |
Microstructure of Finished Tube | Grain size grade 7, tiny holes ≤ 0.2%, unwanted particles ≤ 0.2% |
Wall Thickness Tolerance | Maintained within 0.01 mm for precise measurements |
Surface Roughness (Ra) | ≤ 0.1 μm to prevent rust and ensure smoothness |
You benefit from these controls because they ensure nitinol tubing meets the demands of advanced medical devices.
Laser Cutting
Next, you shape the nitinol tubing using laser cutting. This manufacturing step allows you to create intricate patterns and structures needed for stent retrievers. Femtosecond and pulsed fiber lasers provide high precision, minimizing heat-affected zones and preserving the material’s properties. You use laser micrometers and optical comparators for testing, verifying that dimensional tolerances can be as tight as ±0.005 mm. This precision ensures the devices function reliably inside the body. Laser cutting also supports microfabrication, which is essential for integrating radiopaque markers that help you visualize the devices during procedures.
Heat Setting
Heat setting gives nitinol tubing its shape memory and superelasticity. You place the tubing on stainless steel fixtures or mandrels and heat it between 250 °C and 600 °C for 1 to 120 minutes. This manufacturing step determines the final transformation temperatures and mechanical properties. You use differential scanning calorimetry and tensile testing to monitor changes in the material. Careful control of heat treatment ensures the tubing will expand and contract as needed inside the body. Studies show that balancing temperature, time, and prior cold work is key to achieving optimal performance in medical devices.
Surface Finishing
Surface finishing is the final manufacturing step. You polish and clean the nitinol tubing to remove any defects and improve biocompatibility. Surface roughness is reduced to Ra 0.1 μm or better, which helps prevent corrosion and ensures smooth interaction with blood and tissue. You perform additional testing, such as cytotoxicity and mechanical performance tests, to confirm the tubing’s safety and durability. Surface chemistry analysis shows that proper finishing increases oxygen content, which supports long-term biocompatibility. These steps help you meet the strict requirements for CE-marked medical devices.
Tip: Each stage of manufacturing, from tube drawing to surface finishing, includes multiple rounds of testing to guarantee that nitinol tubing meets the highest standards for safety and performance in medical devices.
Applications in Stents
Clot Engagement
You rely on nitinol tubing to achieve strong clot engagement during thrombectomy. The superelastic properties of nitinol allow the stent to expand inside the vessel and press firmly against the clot. This expansion creates a chronic outward force, which helps the stent struts penetrate and grip the clot. When you deploy a thrombectomy stent, you often leave it expanded for several minutes. This time allows the clot to integrate with the stent, making clot removal more effective.
Quantitative studies show that nitinol surfaces increase platelet coverage by about 54% and fibrin coverage by over 210% after three hours. These changes mean the stent can anchor the clot securely, improving the success rate of clot removal. The high modulus of nitinol also supports strong detachment stress, so you can retrieve the clot with less risk of fragmentation. You see these applications of nitinol tubing in many modern thrombectomy stents, which deliver reliable performance in mechanical thrombectomy.
Vessel Navigation
Navigating tortuous vessels is a challenge in minimally invasive procedures. Nitinol tubing gives you the flexibility and kink resistance needed to move through complex vascular pathways. The shape memory and superelastic properties of nitinol let the stent bend and recover its shape, even after repeated cycles. This flexibility is essential for reaching clots in delicate neurovascular regions.
Experimental data confirm that nitinol tubing maintains over 90% recovery after deformation and achieves a 98% success rate in clinical navigation. The tubing’s thin wall design allows you to access small vessels without sacrificing strength. You can see how nitinol adapts to different vessel diameters and angles, making it ideal for applications in thrombectomy and other minimally invasive procedures.
Radial Force
Radial force is critical for stents used in thrombectomy. Nitinol tubing provides the right balance of flexibility and strength, so the stent can expand against the vessel wall and maintain its position during clot removal. The chronic outward force generated by nitinol ensures that the stent stays open and keeps the vessel clear.
Surface finishing further improves the applications of nitinol tubing by reducing surface roughness and friction. You benefit from smoother stents that move easily inside the body and resist corrosion. The table below highlights key improvements after surface finishing:
Improvement Aspect | Description |
|---|---|
Surface Roughness (Ra <0.1μm) | Electropolishing removes flaws and creates ultra-smooth surfaces. |
Biocompatibility | Smooth surfaces reduce tissue irritation and rejection. |
Nickel Ion Release | Lower nickel ion release enhances safety for long-term use. |
Friction Reduction | Less friction allows easier movement during minimally invasive procedures. |
Device Longevity | Corrosion resistance extends device lifespan. |
Bacterial Adhesion & Sterilization | Smoother surfaces prevent bacteria from sticking, improving sterilization. |
Corrosion Resistance | Better resistance prevents damage over time. |
Manufacturing Quality Control | Advanced polishing ensures consistent high-quality finishes. |
You use nitinol stents in a wide range of applications, especially for mechanical thrombectomy and ischemic stroke treatment. The combination of thin wall design, kink resistance, and optimized radial force makes nitinol tubing the preferred choice for thrombectomy stents in minimally invasive procedures.
Clinical Benefits and Safety
Flexibility and Durability
You depend on nitinol tubing to give stents the flexibility and durability needed for demanding clinical environments. Nitinol’s superelasticity lets you bend and navigate devices through complex vessels without fracture. Mechanical tests show nitinol wires can withstand up to 10 million bending cycles, which means you get long-lasting performance. Surface finishing, such as electropolishing, increases corrosion resistance and reduces imperfections. This process improves torque resistance and minimizes stress points, which helps maintain the quality and safety of your medical devices. Corrosion resistance testing reveals that electropolished nitinol tubing has a much higher breakdown potential, supporting its durability in the body.
Nitinol tubing allows extensive bending without breaking.
Devices maintain flexibility and resist fatigue during repeated use.
Surface finishing enhances both durability and safety.
Patient Outcomes
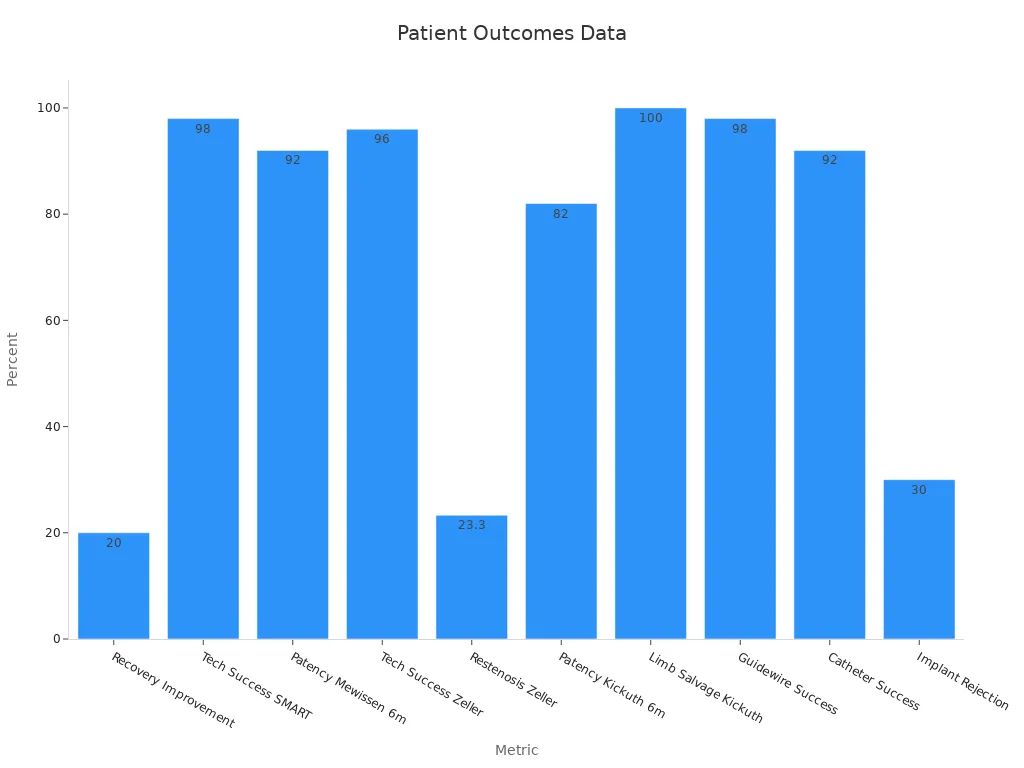
You see direct benefits for patients when you use nitinol tubing in medical devices. Clinical studies show that nitinol stents lead to faster recovery, lower complication rates, and improved long-term outcomes. The advanced properties of nitinol, such as shape memory and biocompatibility, support high technical success rates and device reliability. For example, nitinol stents achieve up to 98% technical success and 92% patency at six months. These results mean you can trust nitinol devices to deliver consistent quality and safety.
Metric | Nitinol Devices | Outcome |
|---|---|---|
Recovery Improvement | 20% | Faster patient recovery |
Complication Rate | Lower | Safer outcomes |
Patency Rate (6 mo) | 92% | Sustained performance |
CE-Marked Device Standards
You must meet strict standards for CE-marked medical devices. Nitinol tubing supports compliance with medical standards by passing rigorous testing for quality, biocompatibility, and durability. Manufacturers follow ISO 9001 and ISO 13485 certifications, which confirm adherence to international standards. Quality control includes fatigue resistance testing, radial force testing, and AI-driven defect detection. You benefit from detailed documentation and biocompatibility testing, which show minimal tissue reactions and no harmful cell activity. These steps ensure your devices meet the highest safety and quality requirements.
Evidence Category | Details |
|---|---|
Regulatory Certifications | ISO 9001:2015, ISO 13485:2016, EU CE marking |
Manufacturing Controls | Monitored temperature, pressure, and alloy composition |
Biocompatibility Testing | Minimal tissue reactions, no significant inflammation |
Quality Control Measures | Fatigue, radial force, and AI-driven defect testing |
Documentation | Detailed records for regulatory review |
Note: You can rely on nitinol tubing to deliver the flexibility, durability, and safety required for CE-marked stents and other medical devices. Strict compliance and quality standards ensure optimal performance and patient outcomes.
You see how nitinol tubing stands at the core of CE-marked stent retrievers, supporting critical applications in modern medicine. Its unique properties and advanced processing enable you to achieve safe, reliable, and effective applications in clot removal. You benefit from tubing that meets strict regulatory standards, ensuring high-quality applications in every procedure. As you explore new applications, you find AI-driven design and miniaturization shaping the future of stent retriever applications. Biomaterial advances and eco-friendly manufacturing practices continue to improve long-term outcomes and support sustainable applications. Collaboration between manufacturers and clinicians drives innovation, expanding the possibilities for future applications in neurosurgery and beyond.
FAQ
What makes nitinol tubing different from stainless steel in stent retrievers?
You get superelasticity and shape memory with nitinol tubing. Stainless steel does not offer these features. Nitinol tubing bends and returns to its shape. This property helps you navigate vessels and remove clots more safely.
How does nitinol tubing improve patient safety?
You benefit from nitinol’s biocompatibility and corrosion resistance. The tubing’s smooth surface reduces tissue irritation. You see fewer complications and better healing.
Note: Nitinol tubing meets strict medical standards for safety.
Can you see nitinol stent retrievers during procedures?
Yes, you can. Manufacturers add radiopaque markers to nitinol tubing. These markers make the device visible under X-ray imaging. You track the stent’s position in real time.
Why do CE-marked stent retrievers use nitinol tubing?
You need devices that meet strict European standards. Nitinol tubing passes quality, durability, and biocompatibility tests.
You get reliable performance
You meet regulatory requirements
You ensure patient safety
How long does nitinol tubing last inside the body?
You can expect nitinol tubing to last for years. The material resists fatigue and corrosion. Clinical data show high durability, even after millions of cycles.
Tip: Proper surface finishing extends the lifespan of nitinol devices.

