Beginner’s Guide to Nitinol Tubing for Acute Ischemic Stroke Thrombectomy
Nitinol tubing stands as a cornerstone in the medical device industry, especially for acute ischemic stroke thrombectomy. This advanced alloy, made from nickel and titanium, offers unique shape memory and superelasticity. Medical device design teams select nitinol tubing for acute ischemic stroke thrombectomy because it enables flexible, durable, and precise devices. The importance of nitinol becomes clear in minimally invasive surgery, where devices must navigate complex vessels with minimal trauma. Medical professionals rely on nitinol tubing for its proven biocompatibility and reliability in surgery. Studies show nitinol tubing improves outcomes in medical procedures, with clinical trials like DAWN reporting 49% functional independence at 90 days using nitinol-based tools, compared to 13% with standard care. Nitinol tubing’s success in medical devices, such as thrombectomy systems, is evident in the following clinical results:
Trial | Device Description | Recanalization Rate | Symptomatic Intracranial Hemorrhage (SICH) Rate | Device-Related Complications Rate |
|---|---|---|---|---|
MERCI | Flexible nitinol wire in helical loops | 46% | 8% | N/A |
Multi MERCI | Same device, IV rt-PA refractory patients | 57% | 10% | 4.5% |
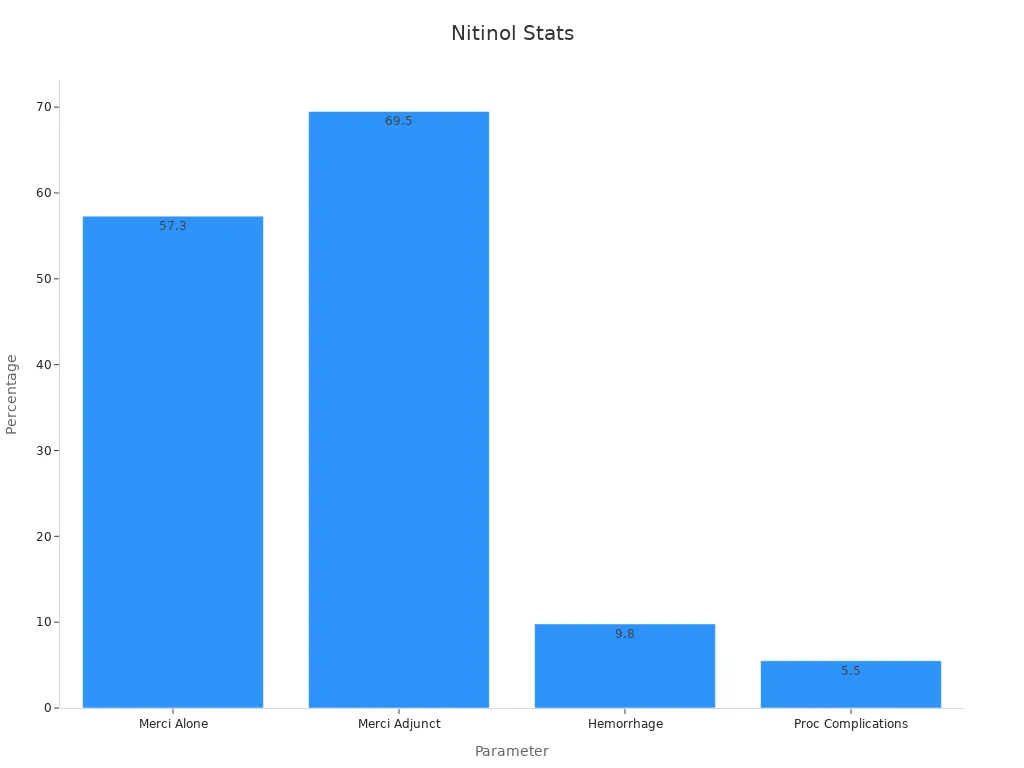
Multi MERCI (confirmed) | Merci system alone / with adjunctive therapies | 57.3% / 69.5% | 9.8% | 5.5% |
For anyone new to this field, this comprehensive guide will clarify why nitinol tubing remains essential for medical devices and minimally invasive surgery.
Key Takeaways
Nitinol tubing offers unique superelasticity and shape memory, allowing medical devices to bend and return to their original shape without damage.
Its biocompatibility and durability make nitinol tubing safe and reliable for use inside the human body, especially in minimally invasive surgeries like stroke thrombectomy.
Nitinol tubing improves thrombectomy device performance by enabling flexible navigation through complex blood vessels, leading to better patient outcomes.
Manufacturing nitinol tubing involves precise alloying, heat treatment, and finishing processes to ensure consistent quality and mechanical properties.
Strict testing and adherence to standards like ASTM and ISO guarantee the safety, effectiveness, and traceability of nitinol tubing in medical devices.
Nitinol Tubing Basics
What Is Nitinol?
Nitinol is a shape memory alloy composed primarily of nickel and titanium. This unique material forms the backbone of many advanced medical devices. Manufacturers produce nitinol tubing by carefully blending these two metals in precise ratios. The resulting alloy exhibits a remarkable ability to change its internal structure in response to temperature or stress. This transformation occurs between two distinct phases: austenite, which has a cubic crystal structure, and martensite, which has a monoclinic structure. The reversible phase transformation between these states gives nitinol tubing its most valuable characteristics.
Nitinol tubing plays a vital role in the medical field. Its microstructure consists of domains of austenite, twinned martensite, and sometimes an intermediate R-phase. Inclusions and dislocation networks, which arise during manufacturing, further influence the mechanical behavior and durability of these tubes. Advanced modeling techniques, such as finite element analysis, help researchers predict how nitinol tubing will perform in demanding medical applications. Medical device manufacturers rely on this data to ensure that nitinol tubing meets the strict requirements of cardiovascular and neural implants.
Medical devices often require tubing that can bend, twist, and recover its shape without permanent damage. Nitinol tubing meets these demands through its unique atomic structure and phase transformation abilities. The production process starts with large cast ingots, which are then drawn down to ultra-fine wires. These wires form the basis for medical tubes used in catheters, stents, and other implantable devices. The superelasticity of nitinol tubing allows it to navigate complex anatomical pathways, making it indispensable in minimally invasive procedures.
Note: Nitinol tubing’s biocompatibility ensures safe integration with human tissue, reducing the risk of rejection or inflammation. Its corrosion resistance and durability make it suitable for long-term medical implants.
Key Properties
Nitinol tubing stands out in the world of medical devices due to two primary properties: superelasticity and shape memory. Superelasticity enables nitinol tubing to undergo significant deformation and return to its original shape without permanent damage. This property proves essential for devices that must flex and adapt to the body’s natural movements. Shape memory allows nitinol tubing to "remember" its original form and revert to it when exposed to specific temperatures. This effect is especially useful in applications where the device must change shape inside the body, such as expanding a stent once it reaches its target location.
The transformation temperature, which controls the switch between martensite and austenite phases, plays a critical role in the performance of nitinol tubing. Manufacturers can adjust this temperature through multi-step heat treatments, tailoring the tubing’s behavior for specific medical applications. Even small changes in the nickel-titanium ratio can significantly affect the transformation temperature and mechanical properties. This level of control allows for the customization of nitinol tubing to meet the exact needs of different medical devices.
Medical applications demand materials that combine flexibility, strength, and reliability. Nitinol tubing delivers on all fronts. Its superelasticity allows it to bend and twist through arteries without permanent deformation. The shape memory effect ensures that devices can be delivered in a compact form and then expand or change shape as needed. Biocompatibility prevents adverse reactions, such as blood clotting, making nitinol tubing safe for use inside the human body. Corrosion resistance and durability further enhance its suitability for long-term implants.
Nitinol tubing enables the development of advanced medical devices for cardiovascular, neural, and orthopedic applications.
The unique combination of superelasticity, shape memory, and biocompatibility sets nitinol tubing apart from other materials.
Applications of nitinol tubing include stents, guidewires, catheters, and other minimally invasive surgical tools.
Experimental studies show that the microstructure of nitinol tubing, including the formation of martensitic variants and the influence of loading direction, directly impacts its mechanical response. Understanding and controlling these factors is crucial for optimizing tubing performance in medical devices. Manufacturers use precise alloy composition control, cold working, and heat treatment to stabilize the desired phase transformation behavior. This ensures that nitinol tubing consistently delivers the flexibility, durability, and reliability required for demanding medical applications.
💡 Tip: Medical device designers should always consider the transformation temperature and microstructural characteristics of nitinol tubing to ensure optimal performance in their specific applications.
Acute Ischemic Stroke Thrombectomy
Stroke Overview
Acute ischemic stroke occurs when a blood clot blocks an artery in the brain, cutting off oxygen and nutrients. This blockage can cause brain cells to die within minutes. Medical professionals recognize two main types of vessel occlusions: proximal large vessel occlusion (PLVO) and distal medium vessel occlusion (DMVO). PLVOs account for about 35–40% of acute ischemic strokes, while DMVOs make up 25–40%. Posterior circulation large vessel occlusions represent 7–12% of cases. Rapid treatment is critical. The STRATIS Registry shows that delays, such as interhospital transfers before thrombectomy, lead to worse outcomes. Hospitals have increased the use of thrombectomy, especially in teaching centers, resulting in more patients returning home after treatment.
Evidence Aspect | Details |
|---|---|
PLVO Prevalence | 35–40% of acute ischemic strokes |
DMVO Prevalence | 25–40% of cases |
Posterior Circulation LVOs | 7–12% of intracranial LVOs |
Impact of Delays | Delayed thrombectomy worsens outcomes |
Thrombectomy Devices
Thrombectomy devices remove clots from blocked vessels, restoring blood flow to the brain. Medical teams use two main types: aspiration catheters and stent-retrievers. Both rely on advanced materials and precise engineering. Mechanical thrombectomy has proven effective and safe for both PLVO and DMVO cases. Recent meta-analyses confirm that these devices improve patient outcomes. Achieving complete recanalization with a single pass, known as the First Pass Effect, leads to better recovery, fewer complications, and lower healthcare costs. Device design continues to evolve, with a focus on improving flexibility, durability, and safety.
Role of Nitinol Tubing
Nitinol tubing for acute ischemic stroke thrombectomy plays a vital role in device performance. Nitinol’s superelasticity and shape memory allow medical devices to navigate complex, tortuous vessels without losing shape or causing trauma. Stent-retriever devices that use nitinol tubing achieve high recanalization rates—about 80%—and favorable clinical outcomes, with up to 40% of patients reaching functional independence at 90 days. Nitinol tubing also reduces vascular injury compared to traditional aspiration devices, as shown by less vessel wall edema in histopathology studies. The unique properties of nitinol tubing enable medical applications that demand both flexibility and strength. Medical device manufacturers select nitinol tubing for acute ischemic stroke thrombectomy because it meets strict requirements for biocompatibility, durability, and performance. These advantages make nitinol tubing a cornerstone in the development of next-generation medical devices for stroke intervention.
Note: The combination of nitinol tubing and innovative device design has transformed applications in acute ischemic stroke care, offering new hope for improved recovery and long-term outcomes.
Nitinol Tubing in Medical Devices
Applications in Minimally Invasive Surgery
Nitinol tubing has become a cornerstone in the design of medical devices for minimally invasive surgery. Medical professionals rely on nitinol tubing for its unique combination of flexibility, strength, and biocompatibility. These properties enable the development of advanced catheters, stents, and other medical device components that must navigate complex anatomical pathways. In urology, several devices highlight the success of nitinol tubing in clinical practice:
The Spring system, a nitinol implant, has shown significant improvement in symptoms for patients with benign prostatic obstruction, with durable results up to 18 months and low adverse event rates.
The ClearRing device, which uses a nitinol compressive ring, improved symptom scores and urine flow rates in a multicenter study, while maintaining a low rate of mild adverse events.
The Butterfly device, a metallic nitinol implant, demonstrated successful catheter removal in patients previously dependent on permanent catheters.
The TIND device, a temporary nitinol implant, provided durable functional improvements for up to three years, with good tolerability and safety.
These examples illustrate the frequency and effectiveness of nitinol tubing in emerging applications in minimally invasive surgery. Medical device manufacturers continue to expand the applications of nitinol tubing, especially in stents and vascular implants, where flexibility and durability are critical.
Benefits for Thrombectomy
Nitinol tubing for acute ischemic stroke thrombectomy offers clear advantages over traditional materials. Medical device applications demand tubing that can maintain shape and resist kinking during complex procedures. Nitinol tubing provides excellent shape memory and kink resistance, which enhances pushability and trackability in tortuous vessels. Compared to stainless steel, nitinol tubing improves navigability in distal vascular territories, making it ideal for catheters and stents used in minimally invasive procedures. The shaft material directly influences procedural efficiency, such as time to clot engagement, and nitinol’s mechanical properties support better outcomes.
The DURABILITY Iliac study supports the long-term safety and effectiveness of nitinol self-expanding stents, reinforcing the value of nitinol tubing in stents and vascular implants. Medical device components made from nitinol tubing demonstrate superior flexibility, biocompatible performance, and durability. These qualities ensure that nitinol tubing for acute ischemic stroke thrombectomy remains essential in modern medical devices and minimally invasive procedures.
Medical device designers select nitinol tubing for its proven ability to combine flexibility, strength, and biocompatibility, supporting a wide range of applications in surgery and device innovation.
Fabrication Process
The manufacturing process of nitinol tubing involves a series of highly controlled steps. Each stage in nitinol processing requires precision to ensure the final product meets the demanding standards of medical device manufacturing. Consistent process control is essential for achieving the superelasticity, shape memory, and biocompatible qualities that make nitinol tubing ideal for acute ischemic stroke thrombectomy devices.
Alloying and Material Prep
Nitinol fabrication begins with alloying, where nickel and titanium are combined in exact proportions. Manufacturers use vacuum-induction melting or vacuum arc re-melting to produce nitinol ingots. These methods help maintain purity and meet ASTM Standard F2063 for chemical composition. The ingots are then forged and rolled at high temperatures into bars or slabs. This step ensures uniformity and prepares the material for further processing. Precision during alloying and material prep directly impacts the microstructure and performance of nitinol tubing.
Drawing and Tube Forming
After alloying, the manufacturing process of nitinol tubing moves to tube forming. Technicians grind round bars and use gun drilling or trepanning to create hollow tubes with high concentricity. These tubes are then sent to redraw houses for further reduction. Tube drawing involves pulling the hollow tubes through dies to achieve the desired outer diameter and wall thickness. The choice of die material, mandrel type, and lubricant all influence the surface finish and mechanical properties. Precision in tube drawing is critical, as it affects the flexibility and fatigue life of the final nitinol tubing.
Heat Treatment and Setting
Heat treatment plays a vital role in nitinol processing. After initial drawing, the tubes undergo full annealing to relieve internal stresses and recrystallize the matrix. Cold work, typically around 30% area reduction, is introduced to develop the desired superelastic or pseudoelastic properties. Final drawing steps further refine the tubing dimensions. Nitinol shape setting occurs during aging treatments, where controlled heating imparts the unique ability to return to a pre-set shape. This step is essential for devices that rely on the shape memory effect, such as stent-retrievers used in thrombectomy.
Tip: Careful control of heat treatment parameters ensures consistent transformation temperatures and reliable superelastic behavior in nitinol tubing.
Laser Cutting and Shaping
Nitinol laser cutting enables the creation of intricate patterns and geometries required for advanced medical devices. Precision laser systems cut the tubing into specific designs, such as the mesh structures found in stent-retrievers. The accuracy of nitinol laser cutting determines the device’s flexibility and ability to navigate complex vascular pathways. Manufacturers often use computer-controlled lasers to achieve micron-level tolerances. This step in the manufacturing process of nitinol tubing allows for rapid prototyping and customization, supporting innovation in medical device manufacturing.
Surface Finishing
Surface finishing enhances both the appearance and performance of nitinol tubing. Centerless grinding removes surface imperfections and ensures the tubing meets exact dimensional specifications. Electropolishing follows, eliminating heat-affected zones created during nitinol laser cutting. This process produces a smooth, defect-free surface that reduces the risk of blood clot formation and improves corrosion resistance. Precision in surface finishing is crucial for maintaining the biocompatible nature of nitinol tubing, especially for long-term implants.
Advanced Joining
Advanced joining techniques connect nitinol tubing to other device components. Methods such as laser welding, brazing, or mechanical joining must preserve the unique properties of nitinol. Precision during joining prevents the introduction of defects that could compromise device performance. Manufacturers often use specialized fixtures and process controls to maintain alignment and ensure strong, reliable joints. Advanced joining supports the integration of nitinol tubing into complex assemblies required for acute ischemic stroke thrombectomy devices.
Consistent process control at every stage of nitinol fabrication ensures that the tubing delivers the required superelasticity, durability, and safety for medical device manufacturing. Statistical studies link process parameters, such as die selection and annealing environment, to the fatigue life and reliability of nitinol tubing. Manufacturers reference ASTM standards and employ rigorous quality checks to maintain high standards throughout nitinol processing.
Quality and Standards
Testing and Inspection
Manufacturers of medical devices rely on rigorous testing and inspection protocols to guarantee the quality of nitinol tubing. Each batch undergoes a series of evaluations to confirm that it meets strict requirements for strength, flexibility, and durability. Differential Scanning Calorimetry (DSC) measures transformation temperatures, while Bend Free Recovery (BFR) tests assess shape recovery and superelasticity. Tensile strength testing validates the tubing’s ability to withstand stress without permanent deformation. Thermal analysis confirms the shape memory and superelastic properties essential for medical applications. Long-term fatigue resistance and corrosion resistance testing ensure that nitinol tubing remains reliable in physiological environments. Clinical studies have shown that nitinol devices maintain excellent biocompatibility and minimal adverse reactions for over a decade.
Test Method | Purpose |
|---|---|
Differential Scanning Calorimetry (DSC) | Measures transformation temperatures, including Austenite Finish (Af) temperature. |
Bend Free Recovery (BFR) | Assesses shape recovery after deformation, indicating superelasticity and shape memory. |
Tensile Strength Testing | Validates material strength to withstand stress without permanent deformation. |
Thermal Analysis | Confirms shape memory and superelastic properties through temperature-related behavior. |
Regulatory Compliance
Medical devices that use nitinol tubing must comply with international standards and regulatory requirements. ASTM F2063 defines the chemical composition, mechanical properties, and testing protocols for nitinol. ISO 10993 specifies biocompatibility testing, including cytotoxicity, sensitization, irritation, systemic toxicity, and genotoxicity. ISO 13485 ensures that manufacturers maintain robust quality management systems for medical device production. FDA clearance confirms that devices meet safety and effectiveness standards before entering the market. Reputable suppliers, such as AccuPath, implement advanced manufacturing techniques and adhere to these standards, ensuring consistent quality and regulatory acceptance.
Regulatory Standards | Role |
|---|---|
ASTM F2063 | Defines chemical composition, mechanical properties, and testing protocols for nitinol. |
ISO 10993 | Specifies biocompatibility testing for medical devices. |
ISO 13485 | Ensures quality management systems for medical device manufacturing. |
FDA Clearance | Regulatory approval process ensuring safety and effectiveness of medical devices. |
Note: Compliance with these standards supports patient safety and device reliability in clinical practice.
Traceability
Traceability plays a critical role in the quality assurance of nitinol tubing for medical devices. Manufacturers document each step of the production process, from raw material sourcing to final inspection. This documentation allows for complete traceability, ensuring that every tube can be tracked back to its origin. Precision in record-keeping helps identify and address any issues quickly, supporting recalls or investigations if necessary. Traceability also demonstrates a commitment to transparency and accountability, which is essential for regulatory approval and long-term trust in medical device manufacturing.
Choosing Nitinol Tubing
Supplier Selection
Selecting the right supplier for nitinol tubing is a critical step in device development. Leading suppliers demonstrate expertise in nitinol processing and maintain strict quality controls. They provide detailed documentation for every batch, ensuring traceability from raw material to finished product. Medical device teams should request references and review supplier certifications. A reputable supplier will offer consistent nitinol tubing with reliable transformation temperatures and mechanical properties. Many top suppliers also support rapid prototyping, which helps accelerate device innovation.
Tip: Always verify that the supplier follows ASTM and ISO standards for nitinol tubing production.
Customization
Customization plays a key role in meeting the unique demands of acute ischemic stroke thrombectomy devices. Device designers often require nitinol tubing with specific diameters, wall thicknesses, and transformation temperatures. Suppliers with advanced manufacturing capabilities can deliver tubing tailored to these requirements. Precision in customization ensures that the tubing will perform as intended during complex procedures. Some suppliers offer laser cutting, shape setting, and surface finishing services. These options allow for the creation of intricate device components with high precision.
Customization Option | Benefit |
|---|---|
Diameter/Wall Control | Matches device specifications |
Transformation Temp | Optimizes device performance |
Surface Finishing | Enhances biocompatibility |
Laser Cutting | Enables complex geometries |
Quality Assurance
Quality assurance guarantees that nitinol tubing meets the highest standards for safety and performance. Reputable suppliers implement rigorous inspection protocols at every stage of production. They use advanced testing methods to verify superelasticity, shape memory, and fatigue resistance. Precision in measurement and documentation supports consistent results. Medical device manufacturers should review quality certificates and audit supplier processes. Reliable nitinol tubing reduces the risk of device failure and supports positive patient outcomes.
Note: Consistent quality assurance and precision in manufacturing help ensure that nitinol tubing will perform reliably in life-saving procedures.
Nitinol tubing continues to transform surgery by enabling advanced applications in thrombectomy and other minimally invasive surgery procedures. Industry reports highlight rapid market growth, driven by technological advancements and new applications. Understanding fabrication and quality control ensures device reliability and patient safety. Beginners should consult experts and reputable suppliers when selecting nitinol tubing for surgery. For those interested in the latest applications, ongoing research and innovation offer new opportunities to improve outcomes.
Explore new trends in nitinol tubing for future medical device applications.
Seek guidance from experienced professionals to ensure optimal device performance.
FAQ
What makes nitinol tubing unique in the medical device industry?
Nitinol tubing stands out due to its superelasticity and shape memory. These properties allow devices to flex and recover shape, which supports innovation in the medical device industry and improves patient outcomes.
How does nitinol tubing support medical device design for thrombectomy?
Engineers use nitinol tubing in medical device design because it provides flexibility, durability, and biocompatibility. These qualities help devices navigate blood vessels safely and efficiently during thrombectomy procedures.
Is nitinol tubing safe for use inside the human body?
Nitinol tubing offers excellent biocompatibility and corrosion resistance. Clinical studies show that it performs safely in long-term implants, making it a trusted material in the medical device industry.
Can nitinol tubing be customized for specific procedures?
Manufacturers can customize nitinol tubing by adjusting diameter, wall thickness, and transformation temperature. This flexibility allows medical device design teams to create devices tailored to unique clinical needs.
What quality standards apply to nitinol tubing?
Manufacturers follow strict standards, such as ASTM F2063 and ISO 13485, to ensure nitinol tubing meets safety and performance requirements. These standards support reliability in the medical device industry.

