How Nitinol Stent Tubing Delivers Superior Flexibility for Thrombectomy

Nitinol stent tubing for mechanical thrombectomy procedures stands apart due to superelasticity, shape memory, and biocompatibility. These properties allow stents to navigate tortuous neurovascular anatomy, minimizing vessel trauma and enhancing clot retrieval. Nitinol stents with helical designs distribute strain evenly during vessel bends, reducing kinking and luminal compromise—a 10-mm tube bent 90° demonstrates a 27% length difference, which nitinol accommodates with ease. Clinical platforms report only a 1.2% limb occlusion rate at one year, even in small neurovascular vessels, underscoring the transformative impact on neurovascular care. Nitinol stent tubing for mechanical thrombectomy procedures delivers unmatched flexibility, safety, and durability in neurovascular care.
Key Takeaways
Nitinol stent tubing offers unmatched flexibility and durability thanks to its superelasticity and shape memory, allowing it to bend through complex vessels without damage.
The shape memory property helps nitinol stents expand precisely inside vessels, improving clot removal and reducing vessel injury during thrombectomy.
Nitinol stents are highly biocompatible, minimizing inflammation and ensuring safe long-term use in delicate neurovascular environments.
Advanced manufacturing techniques enable customization of nitinol stents to fit patient anatomy, enhancing procedural success and patient outcomes.
Clinical studies show nitinol stents deliver high success rates, low complication risks, and better vessel healing compared to traditional metal stents.
Nitinol Stent Tubing for Mechanical Thrombectomy Procedures
Superelasticity
Nitinol stent tubing for mechanical thrombectomy procedures relies on superelasticity to deliver unmatched flexibility and resilience. Superelasticity allows nitinol to undergo significant deformation and return to its original shape without permanent damage. This property is essential for stents navigating the complex, tortuous neurovascular pathways encountered during thrombectomy. Unlike traditional metals, nitinol can bend and flex through sharp vessel turns, maintaining structural integrity and reducing the risk of kinking or collapse.
Clinical studies demonstrate that nitinol stents can endure up to 10 million fatigue cycles under physiological strain, ensuring long-term durability in neurovascular environments. Bench testing confirms that nitinol stent tubing for mechanical thrombectomy procedures maintains effective suction and clot retrieval even after repeated deformation. This resilience supports reliable device performance and minimizes the risk of vessel trauma.
A table below highlights measurable performance benefits of nitinol’s superelasticity in vascular applications:
Measurable Benefit | Description | Quantitative Impact |
|---|---|---|
Fatigue Endurance | Withstands millions of cycles without failure | Up to 10^7 cycles at 0.5–2.9% strain |
Flexibility | Navigates complex neurovascular anatomies | Smooth passage, reduced trauma, shorter procedures |
Resistance to Kinking | Maintains shape under repeated deformation | Prevents permanent deformation |
Reduced Vessel Injury | Minimizes trauma to neurovascular vessels | Lower acute arterial injury rates |
Long-Term Patency | Sustains vessel openness | Higher patency rates than traditional stents |
Superelasticity, combined with shape memory and superelasticity, ensures that nitinol stent tubing for mechanical thrombectomy procedures adapts to the dynamic neurovascular environment, supporting both safety and procedural success.
Shape Memory
Shape memory is a defining characteristic of nitinol, enabling stents to recover their pre-set geometry after deformation. This property is activated by body temperature, allowing nitinol stent tubing for mechanical thrombectomy procedures to be compressed for insertion and then expand precisely within the neurovascular system. Shape memory and superelasticity work together to facilitate smooth navigation through narrow, tortuous vessels, ensuring optimal device deployment and clot engagement.
Stents fabricated from nitinol exhibit consistent expansion and radial force, as demonstrated in clinical studies where devices expanded on average 60.9% beyond the target vessel diameter. This adaptability allows stents to conform to varying vessel sizes and shapes, improving clot retrieval and reducing the risk of vessel wall injury. The MERCI Retriever, the first FDA-approved clot retriever, uses memory-shaped nitinol to transition from a straight to a helical form, enabling effective clot capture even in challenging neurovascular anatomy.
Shape memory and superelasticity also support the development of advanced stent designs, such as those with micro-patterned surfaces, which enhance clot retrieval efficiency without compromising device integrity. These innovations contribute to high recanalization rates and improved neurological outcomes for patients undergoing thrombectomy.
Biocompatibility
Biocompatibility is critical for any material used in neurovascular interventions. Nitinol stent tubing for mechanical thrombectomy procedures demonstrates excellent biocompatibility, minimizing inflammatory responses and reducing the risk of adverse reactions. In vivo studies show that nitinol induces a low immune cell response, with no evidence of necrosis, granulomas, or soft tissue calcification after long-term implantation. Neural and perineural tissues remain non-toxic and non-irritated, supporting the safe use of nitinol in neurovascular applications.
Surface modification techniques, such as electrochemical treatments and thin TiO2-x coatings, further enhance biocompatibility and corrosion resistance by reducing nickel release while preserving shape memory and superelasticity. These modifications ensure that nitinol stents maintain their functional advantages without compromising patient safety.
Research on nitinol-based devices coated with bioengineered matrices and heparin demonstrates reduced thrombogencity and transient immune responses, with full tissue integration over time. These findings confirm that nitinol stent tubing for mechanical thrombectomy procedures offers superior biocompatibility, supporting long-term vessel patency and reducing the risk of complications.
Biocompatibility, combined with shape memory and superelasticity, positions nitinol as the material of choice for neurovascular stents. Its ability to maintain performance under physiological conditions, resist corrosion, and minimize inflammatory responses ensures optimal outcomes for patients undergoing thrombectomy.
Procedural Benefits
Vessel Navigation
Nitinol stents provide unmatched flexibility for navigating the complex and tortuous pathways of the neurovascular system. The superelastic properties of nitinol allow these stents to bend and conform to sharp vessel curves without losing their structural integrity. This flexibility reduces the risk of vessel trauma and enables physicians to reach distal occlusions that would challenge traditional devices. Neurovascular procedures often require devices to traverse vessels with varying diameters and acute angles. Nitinol stents adapt to these changes, maintaining a smooth profile and minimizing the risk of kinking or collapse. The ability to maintain lumen patency during navigation supports both procedural safety and efficiency, especially in time-sensitive thrombectomy cases.
Clot Engagement
Effective clot removal depends on the stent’s ability to integrate with and capture thrombus material. Nitinol stents, especially those with hybrid cell designs, demonstrate improved clot engagement in clinical settings. For example, a multicenter observational study compared the Aperio Hybrid stent, which uses nitinol tubing with a combination of closed and open cells, to the Solitaire FR/X stent. The Aperio Hybrid’s design allows it to adjust its length to match both thrombus size and vessel tortuosity. This adaptability leads to a trend toward higher recanalization rates and fewer device passes, suggesting greater efficiency in clot capture. The nitinol tube, combined with a dense platinum core, also enhances fluoroscopic visibility, supporting enhanced precision in neurovascular procedures. These features contribute to more reliable and effective clot removal, reducing procedure time and improving patient outcomes.
Vessel Apposition
Proper vessel wall apposition is critical for the success of neurovascular stents. Nitinol stents exhibit excellent conformability, allowing them to maintain close contact with the vessel wall even in irregular or tapered vessels. Studies using 3D fusion imaging have assessed stent apposition in patients treated with LVIS nitinol stents. Results show that approximately 25% of patients experienced a crescent sign malapposition, while about 50% exhibited edge malapposition. Comparisons with other nitinol laser-cut stents, such as Enterprise2 and Neuroform EZ, revealed varying rates of malapposition, highlighting the importance of stent design and sizing. Computational modeling further demonstrates that an optimal stent-to-artery diameter ratio between 1.2 and 1.4 maximizes lumen gain and minimizes malapposition-related complications. Poor apposition can lead to disturbed blood flow, delayed stent incorporation, and increased risk of thrombosis. The Pipeline Embolization Device, a nitinol flow diverter, undergoes structural remodeling after deployment, with changes in diameter and length affecting treatment efficacy. These findings underscore the need for precise sizing and deployment techniques to ensure optimal vessel wall apposition and long-term neurovascular health.
Proper apposition of nitinol stents not only supports immediate procedural success but also reduces the risk of complications such as in-stent thrombosis and the need for additional antiplatelet therapy.
Fatigue Resistance
Durability and reliability are essential for devices used in neurovascular interventions. Nitinol stents excel in fatigue resistance, withstanding millions of deformation cycles without structural failure. This resilience is especially important during repeated device manipulation and repositioning, which are common in complex thrombectomy procedures. The superelastic nature of nitinol allows stents to recover their original shape after each cycle, maintaining their effectiveness throughout the procedure. Fatigue resistance also contributes to long-term vessel patency, as stents remain functional and stable even under continuous physiological stress. Physicians can rely on nitinol stents for consistent performance, reducing the likelihood of device-related complications and supporting better patient outcomes.
Key advantages of nitinol stents in neurovascular procedures:
Reliable navigation through tortuous anatomy
Efficient and effective clot removal
Superior vessel wall apposition
Long-term durability and fatigue resistance
Nitinol stents set the standard for safety, flexibility, and performance in neurovascular thrombectomy, enabling clinicians to achieve optimal results in challenging cases.
Customizable Nitinol Stents
Microfabrication
Advanced microfabrication techniques empower manufacturers to create customizable nitinol stents that meet specific procedural needs. Methods such as magnetron sputtering, UV lithography, and wet etching enable high structural accuracy and smooth edge profiles. These processes allow for layer thicknesses up to 75 µm and support the creation of complex geometries that traditional manufacturing cannot achieve. Dimensional accuracy remains high, with less than 5% variation in nitinol spring diameters ranging from 0.7 to 1.9 mm. Manufacturers use sacrificial copper fixtures and polymeric cores to shape nitinol wires, reducing costs and turnaround times. Rapid prototyping capabilities allow for patient-specific implant fabrication, improving procedural outcomes and supporting the trend toward customizing nitinol stents for individual anatomy.
Metric / Feature | Description / Value |
|---|---|
Fabrication method | Magnetron sputtering, UV lithography, wet etching |
Structural accuracy | High, with smooth edge profiles |
Layer thickness | Up to 75 µm |
Dimensional accuracy | <5% variation in spring diameter (0.7–1.9 mm) |
Rapid prototyping | Enables patient-specific stent fabrication |
Complexity of geometries | Supports intricate shapes and multifunctional integration |
Design Optimization
Design optimization plays a crucial role in the performance of customizable nitinol stents. Engineers use computational tools like finite element analysis and parametric CAD modeling to refine stent geometry. These methods evaluate mechanical properties such as flexibility, radial force, and fatigue resistance. For example, three-point bending tests following ASTM standards reveal that lower force-displacement values indicate higher flexibility. Open-cell, closed-cell, and hybrid stent designs each display unique mechanical behaviors, with optimized models balancing flexibility and radial strength. Manufacturing processes such as laser machining, chemical polishing, and precise heat treatment further enhance mechanical stability and surface quality. By adjusting strut thickness, surface patterns, and overall design, manufacturers can tailor stents to maximize clot retrieval and minimize complications.
Design optimization benchmarks ensure that customizable nitinol stents deliver consistent performance and adapt to the demands of neurovascular procedures.
Tailored Performance
Customizing nitinol stents allows clinicians to match device characteristics to patient anatomy and procedural requirements. Engineering studies show that adjusting wall thickness and diameter, as well as applying specialized surface treatments, improves flexibility and biocompatibility. Accelerated fatigue testing confirms that nitinol stents withstand millions of stress cycles, reducing the risk of fracture and ensuring long-term vessel patency. Shape-memory and superelastic properties enable stents to navigate complex vascular pathways without permanent deformation, enhancing procedural safety. Finite Element Analysis predicts device behavior and fatigue properties before prototyping, supporting safer and more reliable stent deployment.
Clinical data highlight the effectiveness of customizable nitinol stents. Technical success rates in aneurysm treatment reach up to 97%, with low complication rates and high long-term occlusion. In thrombectomy, TIMI flow restoration rates exceed 90%, and good functional outcomes are observed in nearly half of patients at three months. The chart below illustrates these clinical outcomes:
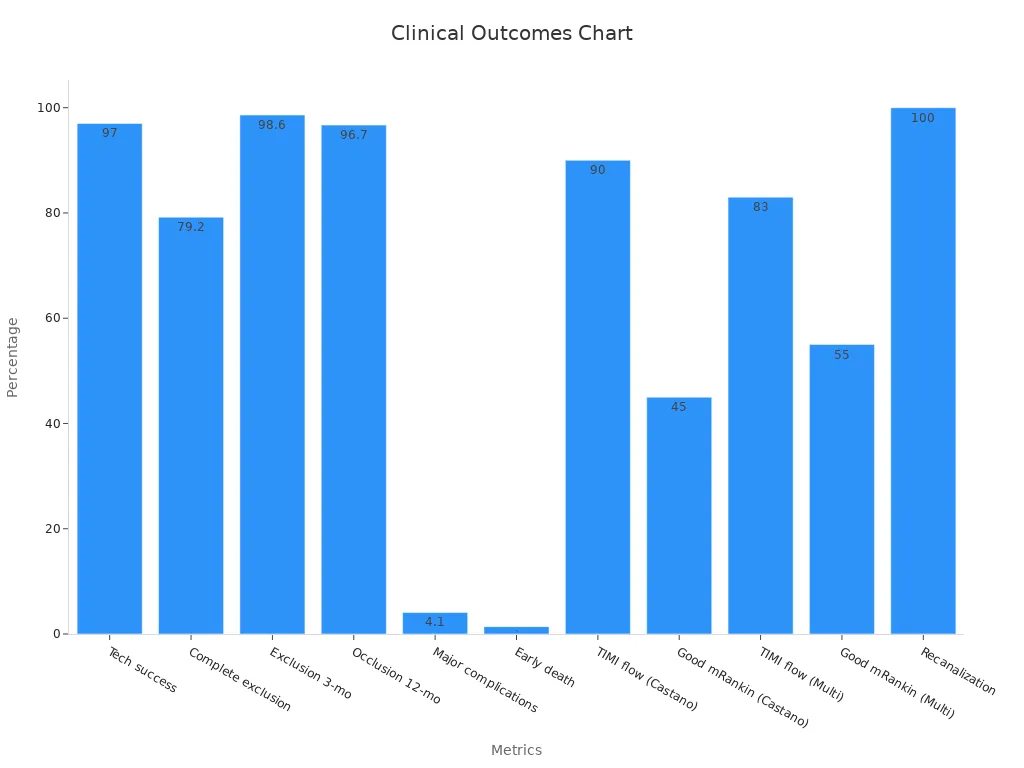
Customizing nitinol stents through advanced manufacturing and design optimization ensures that each device meets the highest standards for safety, flexibility, and effectiveness in neurovascular interventions.
Material Comparison
Traditional Metals
Stainless steel and cobalt-chromium have served as the foundation for vascular stents for decades. These metals provide strength and some degree of flexibility, but they lack the advanced properties needed for modern neurovascular interventions. Stainless steel stents often appear stiffer, which can limit their ability to conform to the intricate curves of cerebral vessels. Cobalt-chromium alloys offer higher strength, but their reduced flexibility and workability can challenge precise placement, especially in procedures involving aneurysms or tortuous anatomy. Both materials show good corrosion resistance, yet they may release metal ions over time, raising concerns about long-term biocompatibility in sensitive neurovascular environments.
Flexibility and Durability
Nitinol stents outperform traditional metals in both flexibility and durability. The superelasticity and shape memory of nitinol allow stents to bend, twist, and return to their original shape without permanent deformation. This property proves essential for navigating the complex pathways encountered during the treatment of aneurysms and in thrombectomy devices. In contrast, stainless steel and cobalt-chromium stents risk kinking or fracturing under repeated stress. Fatigue testing demonstrates that nitinol stents withstand millions of deformation cycles, maintaining their structure and function even after extensive use. The superior corrosion resistance of nitinol further reduces the risk of adverse reactions and ensures long-term vessel compatibility. These advantages directly translate to safer, more effective treatment of aneurysms and improved outcomes for patients.
Property | Stainless Steel | Cobalt-Chromium | Nitinol |
|---|---|---|---|
Flexibility | Moderate | Low | High |
Fatigue Resistance | Moderate | Moderate | Exceptional |
Biocompatibility | Good | Good | Excellent |
Shape Memory | None | None | Present |
MRI Compatibility | Limited | Limited | Excellent |
Clinical Preference
Clinicians increasingly prefer nitinol stents for neurovascular procedures, especially when treating aneurysms or performing mechanical thrombectomy. The ability of nitinol stents to self-expand and conform to vessel walls reduces the risk of malapposition and improves blood flow restoration. Clinical trials such as MISAGO 1 and ORION report superior patient outcomes and lower complication rates with nitinol stents compared to those made from traditional metals. Nitinol stents also demonstrate less acute recoil after deployment, providing more reliable vessel support. Their non-ferromagnetic nature ensures compatibility with MRI, which is critical for follow-up imaging in patients with aneurysms. The combination of flexibility, durability, and safety makes nitinol the material of choice for modern thrombectomy devices and for the management of complex aneurysms.
The evolution from stainless steel and cobalt-chromium to nitinol stents marks a significant advancement in the treatment of aneurysms and acute ischemic stroke. This shift reflects the growing demand for devices that offer superior flexibility, long-term durability, and optimal patient outcomes.
Clinical Evidence
Outcomes
Clinical studies consistently show that stents deliver strong results in neurovascular care. Physicians report high technical success rates, with up to 97% of procedures achieving their intended goals. Recanalization rates reach 100% in some studies, meaning blood flow is restored in nearly every case. Patients experience significant improvements in symptoms and quality of life. For example:
A single-center analysis of 1,094 stents in 406 patients found primary patency rates of 57.3% at five years, with secondary patency reaching 80.9%.
Vein diameters increased after stent placement, showing effective restoration of vessel lumen.
Patients with chronic deep vein thrombosis saw Villalta scores drop from 15.7 to 7.4, reflecting better clinical outcomes.
The BLIND study reported 3-year patency rates of 90.3% and freedom from reintervention at 92.6%.
These results highlight the long-term durability and effectiveness of stents in neurovascular care, especially for the treatment of cerebral aneurysms and related conditions.
Safety Data
Stents undergo rigorous safety testing before clinical use. Manufacturers follow ISO 10993 standards, which require chemical, biological, and mechanical evaluations. Tests confirm that stents resist corrosion, do not release harmful levels of nickel, and remain stable in the body. Hemocompatibility studies show that stents do not trigger blood clotting or damage blood cells. Genotoxicity and carcinogenicity tests confirm no DNA damage or cancer risk. In preclinical animal studies, stents show rapid tissue coverage, no migration, and no negative effects on vessel healing. Major complication rates remain low, with perioperative mortality under 1% and major complications at 3.7%. These findings support the safety of stents in neurovascular care, including the management of aneurysms.
Real-World Cases
Real-world applications of nitinol stents demonstrate their value in neurovascular care. Devices like the EMBOTRAP stent enable faster clot removal and better patient recovery. Published reports show that 55% of patients achieve good functional recovery after stent placement, with major complications at only 4.1% and early mortality at 1.4%. Stents maintain flexibility and resist fracture, reducing the risk of vessel injury and restenosis. Intravascular ultrasound imaging confirms that stents increase vessel diameter and improve blood flow. Long-term studies reveal sustained patency and limb salvage benefits, even after five years. The chart below illustrates primary patency rates over time from several major studies:
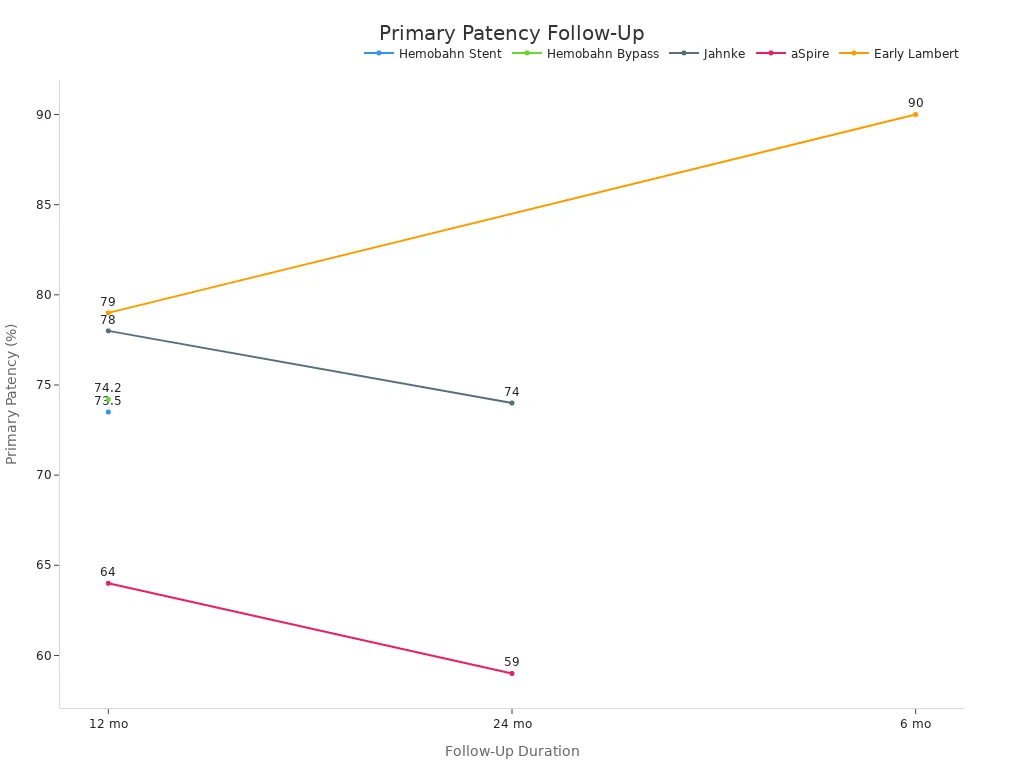
Stents play a critical role in neurovascular care, especially in the treatment of aneurysms. Their proven safety, durability, and effectiveness make them the preferred choice for clinicians seeking the best outcomes for their patients.
Stents with advanced design features continue to transform thrombectomy procedures. Recent innovations include laser-cut stents that reduce foreshortening and improve landing precision. Clinicians see better preservation of venous confluences and improved outcomes, as shown by rising rates of first-pass reperfusion and complete vessel restoration.
Study/Author (Year) | Focus/Contribution | Key Findings Related to Stent Properties and Procedural Advantages |
|---|---|---|
Dyet et al. (2000) | Assessment of radial force, flexibility, radio-opacity, and trackability | Demonstrated key procedural advantages of stents such as flexibility and visibility under imaging |
Kleinstreuer et al. (2008) | Finite element analysis on stent-grafts | Validated mechanical behavior of stents under physiological conditions |
Ongoing advancements in stent architecture and material science will further improve thrombectomy success and patient outcomes.
FAQ
What makes nitinol stent tubing more flexible than stainless steel?
Nitinol’s superelasticity allows it to bend and return to its original shape. Stainless steel lacks this property. Nitinol adapts to vessel curves without kinking, which improves navigation and safety during thrombectomy.
How does nitinol stent tubing reduce vessel trauma?
Nitinol stents conform to vessel walls and flex with movement. This reduces pressure points and minimizes injury. Physicians report fewer complications and better vessel healing with nitinol compared to traditional metals.
Can nitinol stents be used in MRI procedures?
Yes. Nitinol is non-ferromagnetic and safe for MRI imaging. Patients with nitinol stents can undergo MRI scans without risk of device movement or image distortion.
Are nitinol stents customizable for different patients?
Manufacturers use advanced microfabrication to tailor nitinol stents. They adjust strut thickness, diameter, and surface patterns to match patient anatomy and procedural needs. This customization improves outcomes and device performance.

