What Are Integrated Balloon and Catheter OEM Solutions
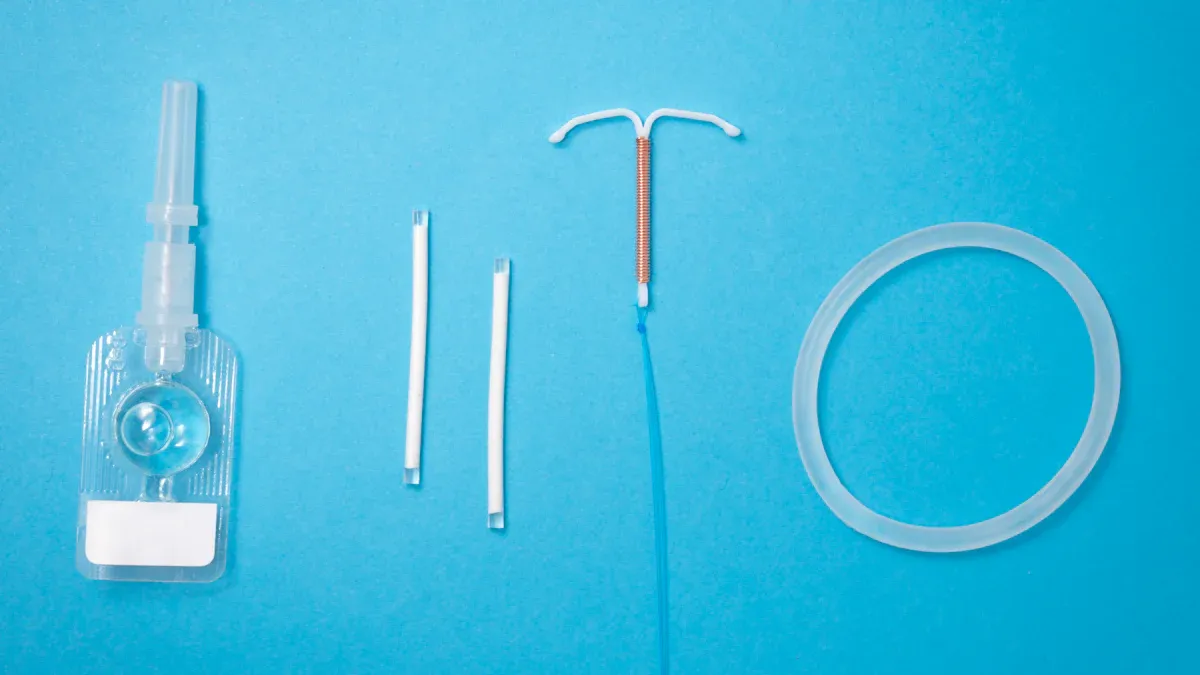
Integrated balloon and catheter OEM solutions refer to the seamless development and manufacturing of medical devices that combine balloons and catheters for specialized healthcare applications. These solutions play a pivotal role in the medical device industry by addressing the growing demand for minimally invasive procedures.
The global ultra-high pressure balloon catheter market was valued at USD 453.8 million in 2024.
It is projected to grow at a CAGR of 9.42% from 2025 to 2030, driven by the rising prevalence of cardiovascular diseases and the adoption of advanced technologies.
Such innovations enable healthcare providers to meet specific medical needs, improving patient outcomes and advancing treatment options.
Key Takeaways
Integrated balloon and catheter solutions help patients by offering custom devices.
These solutions make manufacturing easier, cut costs, and speed up delivery.
Custom designs make treatments more accurate, improving results for patients.
Special tests, like leak tests, ensure balloon catheters are safe to use.
Working with skilled companies like Accupath gives access to trusted devices.
Using new technology in factories saves time and makes better products.
Doctors get the newest tools by teaming up with OEM partners, boosting care.
Following rules and checking quality is key for making and selling devices.
Understanding Integrated Balloon and Catheter OEM Solutions
What Are Integrated Balloon and Catheter OEM Solutions?
Integrated balloon and catheter OEM solutions represent a comprehensive approach to designing and manufacturing medical devices that combine balloons and catheters. These solutions address the need for precision, reliability, and customization in the healthcare industry. Companies like Teleflex specialize in offering end-to-end capabilities, including concept design, material selection, rapid prototyping, and testing. This integrated process reduces development costs and accelerates time-to-market, ensuring that innovative devices reach patients faster.
Key characteristics of these solutions include advanced testing methods such as burst-to-failure and compliance measurement. These tests validate performance and ensure safety. Additionally, customization plays a central role, allowing manufacturers to tailor devices to specific medical applications. For example, Accupath's Vertebral Balloon Catheter demonstrates how integrated solutions can meet specialized needs in spinal surgeries. By combining high-pressure resistance with customizable dimensions, this product exemplifies the potential of integrated balloon and catheter OEM solutions.
The Role of OEMs in Medical Device Manufacturing
OEMs serve as the backbone of the medical device industry by transforming raw materials into essential components. They provide the expertise and infrastructure needed to produce high-quality devices efficiently. Outsourcing manufacturing to OEMs enables healthcare companies to focus on innovation while ensuring compliance with regulatory standards. This collaboration accelerates the transition from design to delivery, allowing life-saving technologies to reach patients more quickly.
For instance, OEMs like Teleflex contribute to the supply chain by offering precision manufacturing capabilities. They measure critical parameters such as catheter dimensions and balloon inflation behavior with specialized equipment. This attention to detail ensures that devices meet stringent performance and safety requirements. By partnering with experienced OEMs, medical companies can enhance their production processes and deliver reliable products to the market.
Importance in the Medical Industry
Integrated balloon and catheter OEM solutions play a vital role in advancing medical technology. They streamline manufacturing processes, reduce costs, and improve patient outcomes. According to a study by the West Health Institute, medical device integration could save the U.S. healthcare system an estimated $30 billion annually by enhancing efficiency and productivity. These solutions also foster innovation by enabling the development of precision devices tailored to specific medical needs.
The benefits extend beyond cost savings. Integrated solutions improve operational efficiency, support informed decision-making, and enhance patient care. For example, real-time access to patient data allows healthcare providers to make accurate diagnoses and develop personalized treatment plans. This approach not only improves outcomes but also strengthens market differentiation by demonstrating a commitment to patient-centric care.
Accupath exemplifies the importance of integrated solutions through its Vertebral Balloon Catheter. This device combines advanced design with robust construction to address the challenges of spinal surgeries. By leveraging integrated balloon and catheter OEM solutions, Accupath continues to lead the way in delivering innovative medical devices that meet the evolving needs of healthcare providers.
Key Components and Features of Integrated Solutions
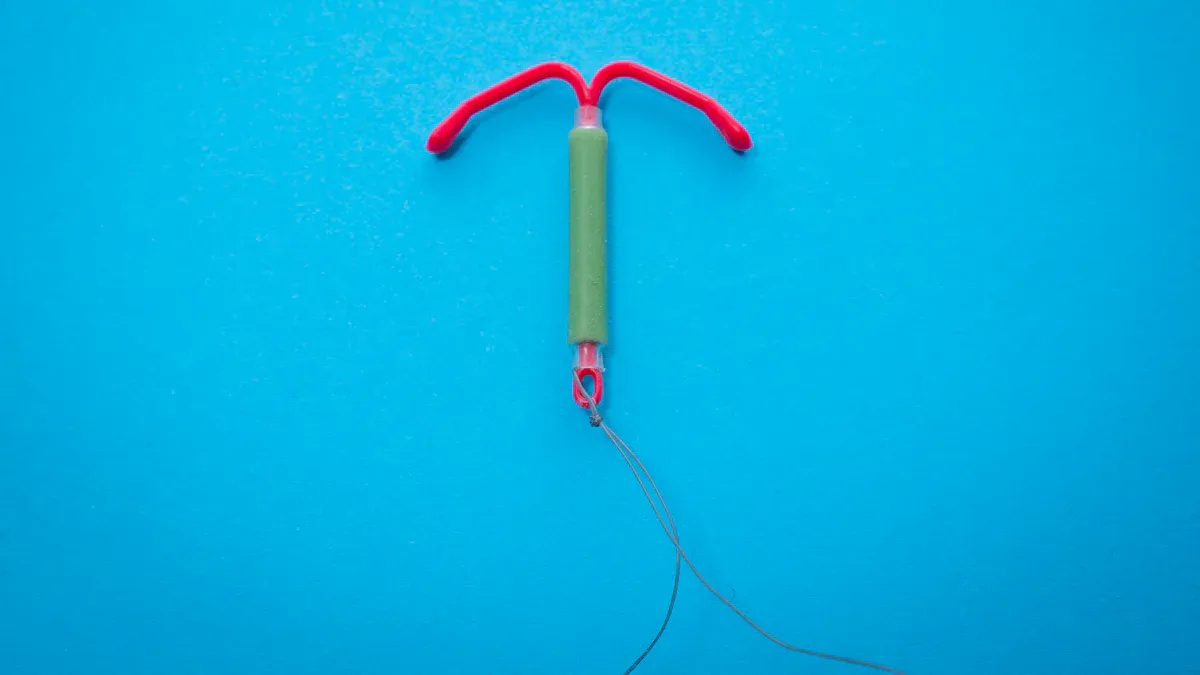
Integrated balloon and catheter OEM solutions rely on several critical components and features to meet the demands of modern medical procedures. These components ensure precision, safety, and adaptability in various healthcare applications.
Types of Medical Balloons
Medical balloons serve diverse purposes in minimally invasive procedures. Each type is designed to address specific clinical needs.
Dilation Balloons
Dilation balloons are essential for expanding narrowed or obstructed pathways within the body. These balloons are commonly used in angioplasty procedures to widen blood vessels and restore proper blood flow. Their design prioritizes controlled inflation to minimize risks during treatment.
Occlusion Balloons
Occlusion balloons temporarily block blood flow or isolate specific areas during surgical procedures. These balloons are vital in preventing excessive bleeding or creating a clear surgical field. Their high-pressure resistance ensures reliability in critical situations.
Drug-Delivery Balloons
Drug-delivery balloons combine therapeutic intervention with medication administration. These balloons release drugs directly to targeted areas, enhancing treatment efficacy. Their design incorporates advanced materials to ensure precise drug delivery without compromising balloon integrity.
Types of Catheters
Catheters play a pivotal role in delivering medical balloons to the desired location. Different types of catheters cater to various diagnostic and therapeutic needs.
Diagnostic Catheters
Diagnostic catheters assist in identifying medical conditions by collecting data or delivering contrast agents. Their design emphasizes trackability and precision to navigate complex anatomical structures.
Therapeutic Catheters
Therapeutic catheters are used to treat conditions such as blockages or lesions. These catheters often feature enhanced pushability and torquability, enabling effective treatment delivery.
Specialty Catheters
Specialty catheters address unique medical challenges, such as those encountered in spinal surgeries. For example, Accupath's custom-engineered catheters, like the Vertebral Balloon Catheter, combine advanced features to meet the specific demands of vertebroplasty and kyphoplasty procedures.
Performance Metric | Description |
|---|---|
Trackability | Measures the ability of the catheter to navigate through vessels. |
Torquability | Assesses the catheter's responsiveness to rotational forces. |
Pushability | Evaluates the force required to advance the catheter through a vessel. |
Balloon Fatigue Testing | Determines the durability of the balloon under repeated inflation and deflation cycles. |
Compliance Measurement | Measures the ability of the balloon to expand under pressure. |
Materials Used in Balloons and Catheters
The choice of materials significantly impacts the performance and safety of balloon catheters.
Common Materials for Balloons
Advancements in polymer technology have introduced materials like nylon and polyester for balloon manufacturing. These materials exhibit excellent mechanical properties, allowing balloons to withstand high pressures during procedures. Their durability ensures patient safety and device reliability.
Common Materials for Catheters
Catheters often utilize materials such as polyurethane and polyethylene. These materials provide flexibility and biocompatibility, ensuring smooth navigation through the body. Their lightweight nature enhances the overall performance of balloon catheters in medical applications.
Note: The integration of high-quality materials and performance metrics ensures that Accupath's balloon catheters meet the rigorous demands of modern healthcare.
The Development Process for Integrated Balloon and Catheter OEM Solutions
Design and Engineering
The design and engineering phase forms the foundation of integrated balloon and catheter OEM solutions. Engineers begin by defining the device's purpose and identifying the specific medical application it will address. This phase involves creating detailed blueprints and 3D models to visualize the product's structure and functionality. Advanced software tools enable precise simulations, allowing engineers to predict how the device will perform under various conditions.
Collaboration between design teams and healthcare professionals ensures that the product meets clinical requirements. For example, Accupath's Vertebral Balloon Catheter demonstrates how thoughtful design can address the challenges of spinal surgeries. Its dual-lumen structure and high-pressure resistance reflect meticulous engineering tailored to specific surgical needs. By prioritizing functionality and safety, the design phase sets the stage for successful product development.
Material Selection
Material selection plays a critical role in the performance and safety of balloon and catheter systems. Engineers evaluate materials based on several key criteria:
Mechanical properties such as surface smoothness, puncture resistance, and burst strength.
Biocompatibility to prevent adverse reactions within the body.
Cost-effectiveness to ensure affordability in medical applications.
The ability to withstand high pressures during procedures for enhanced safety and effectiveness.
These considerations guide the choice of materials like nylon, polyester, polyurethane, and polyethylene. Each material offers unique advantages, such as flexibility, durability, or lightweight properties. For instance, Accupath's Vertebral Balloon Catheter utilizes materials engineered to handle pressures up to 700 pounds, ensuring reliability during vertebroplasty and kyphoplasty procedures. By selecting the right materials, manufacturers enhance both the performance and safety of their devices.
Prototyping and Testing
Prototyping and testing validate the design and material choices made during earlier stages. Engineers create physical prototypes to assess the device's functionality and identify potential improvements. Non-destructive testing methods, such as compliance measurement and balloon fatigue testing, play a crucial role in this phase. These tests evaluate the balloon's ability to expand under pressure and its durability during repeated inflation cycles.
Non-destructive testing ensures that prototypes meet stringent safety and performance standards without compromising their integrity. For example, Accupath employs advanced testing techniques to verify the high-pressure resistance of its Vertebral Balloon Catheter. This rigorous approach minimizes the risk of device failure during medical procedures. By refining prototypes through iterative testing, manufacturers deliver reliable products that meet the demands of modern healthcare.
Balloon Catheter Leak Test System
The balloon catheter leak test system plays a crucial role in ensuring the reliability and safety of medical devices. This system evaluates the integrity of balloon catheters by detecting leaks and assessing their performance under various conditions. Manufacturers rely on highly sensitive leak testing to identify even the smallest defects, ensuring that each device meets stringent quality standards.
Leak testing methods, such as volumetric leak testing and air leak testing systems, are commonly employed during the manufacturing process. These methods measure the sensitivity of the balloon catheter to pressure changes, ensuring that it can withstand the demands of medical procedures. For example, volumetric leak testing evaluates the amount of air or fluid escaping from the catheter, providing precise data on its performance. Similarly, air leak testing systems use pressurized air to detect leaks, offering a non-invasive and efficient solution for quality control.
To maintain accuracy and consistency, manufacturers adhere to international standards. The ISO 10555-4 and ISO 25539-2 guidelines outline specific test methods for balloon catheters. These include rated burst pressure, fatigue testing, and deflation time assessments. The table below highlights key standards used in balloon catheter leak test systems:
Standard | Description |
|---|---|
ISO 10555-4 | Intravascular Catheters – Sterile and single-use – Balloon Dilation Catheters |
Annex A | Test method for balloon rated burst pressure (RBP) |
Annex B | Balloon fatigue test for freedom from leakage and damage on inflation |
Annex C | Deflation time |
Annex D | Test method for balloon diameter to inflation pressure |
Annex E | Balloon material selection guidelines – longitudinal burst with no fragmentation |
ISO 25539-2 | Vascular Stents |
D.5.2.2.3 | Balloon rated burst pressure |
D.5.2.2.4 | Balloon rated fatigue |
Accupath integrates these rigorous standards into its manufacturing processes. For instance, the Vertebral Balloon Catheter undergoes extensive leak testing to ensure its high-pressure resistance and reliability during spinal surgeries. By employing advanced balloon catheter leak test systems, Accupath delivers products that meet the highest levels of safety and performance.
Regulatory Compliance and Quality Assurance
Regulatory compliance and quality assurance are fundamental to the development of balloon catheters. Manufacturers must navigate a complex landscape of regulations to ensure their devices meet global standards. These regulations safeguard patient safety and promote the reliability of medical devices.
Organizations like the FDA and ISO establish guidelines for the design, testing, and manufacturing of balloon catheters. Compliance with these standards involves rigorous documentation, regular audits, and adherence to quality management systems. For example, ISO 13485 certification demonstrates a manufacturer's commitment to producing safe and effective medical devices.
Quality assurance processes include comprehensive testing and inspection at every stage of production. Manufacturers evaluate critical parameters such as balloon diameter, inflation pressure, and material integrity. These measures ensure that each device performs as intended during medical procedures. Accupath exemplifies this commitment by implementing robust quality assurance protocols for its Vertebral Balloon Catheter. This approach guarantees that the product meets the specific needs of healthcare providers while adhering to regulatory requirements.
By prioritizing regulatory compliance and quality assurance, manufacturers like Accupath enhance their reputation and build trust with healthcare professionals. These efforts not only improve patient outcomes but also drive innovation in the medical device industry.
Benefits of Integrated Balloon and Catheter OEM Solutions
Customization for Specific Medical Needs
Integrated balloon and catheter OEM solutions offer unparalleled customization, enabling healthcare providers to address unique medical challenges. These solutions allow manufacturers to design custom-engineered medical components tailored to specific patient needs. For instance, Accupath's Vertebral Balloon Catheter exemplifies this approach by offering customizable balloon diameters and lengths, ensuring precise fit and functionality during spinal surgeries.
Customization enhances patient care by improving the precision and effectiveness of treatments. Tailored devices empower healthcare professionals to deliver targeted interventions, reducing the risk of complications. The table below highlights the quantifiable benefits of customization in integrated solutions:
Benefit | Description |
|---|---|
Improved Patient Care | Tailored monitoring services enhance the precision and effectiveness of care for individual patients. |
Enhanced Efficiency | Integration of telehealth functionality extends healthcare access beyond traditional settings. |
Cost Advantages | Subscription-based models can be more cost-effective for patients, especially those with chronic conditions. |
Empowered Patients | Personalized care options enable patients to take an active role in managing their health. |
By leveraging OEM expertise, manufacturers like Accupath ensure that their products meet the diverse needs of modern healthcare, ultimately improving patient outcomes.
Streamlined Manufacturing Processes
Integrated OEM solutions streamline manufacturing processes by incorporating advanced technologies and methodologies. The adoption of Industry 4.0 technologies, such as IoT, AI, and robotics, has revolutionized production efficiency. These innovations reduce downtime, enhance product quality, and optimize resource utilization. Comparative studies reveal that smart factories utilizing these technologies achieve significant improvements in key performance indicators (KPIs), including production speed and defect rates.
Accupath exemplifies this efficiency through its robust manufacturing processes. By integrating advanced leak testing systems, the company ensures the reliability of its balloon catheters while minimizing production delays. This streamlined approach not only accelerates time-to-market but also reduces costs, making high-quality medical devices more accessible to healthcare providers.
Enhanced Quality and Reliability
Integrated OEM solutions prioritize quality and reliability, ensuring that medical devices meet stringent safety standards. Manufacturers employ rigorous testing protocols, such as balloon fatigue testing and compliance measurement, to validate device performance. These measures minimize the risk of device failure during critical procedures.
Accupath's commitment to quality is evident in its Vertebral Balloon Catheter, which undergoes extensive leak testing to ensure high-pressure resistance and durability. Data enrichment further enhances reliability by filling gaps, refreshing outdated information, and minimizing duplicates. This approach enables faster responses to customer needs and supports informed decision-making.
Additionally, integrated solutions empower users with advanced analytics and AI-driven insights. These tools enhance operational efficiency and scalability, allowing manufacturers to onboard new customers in real time. By partnering with trusted OEMs like Accupath, healthcare providers gain access to reliable, high-performance medical devices that improve patient care.
Cost and Time Efficiency
Integrated balloon and catheter OEM solutions significantly enhance cost and time efficiency in medical device manufacturing. By streamlining processes and leveraging advanced technologies, these solutions reduce production timelines and operational expenses. Manufacturers like Accupath exemplify this efficiency by integrating cutting-edge methodologies into their production workflows.
One of the primary ways OEM solutions save costs is through automation and process optimization. Automation minimizes manual intervention, reducing labor costs and the likelihood of human error. For example, robotic process automation (RPA) has demonstrated remarkable results in various industries. It reduces the need for full-time employees (FTEs), lowers transaction costs, and improves accuracy. The table below highlights key metrics that illustrate these benefits:
Metric | Description | Example |
|---|---|---|
FTE Reduction | Compares the number of employees needed before and after RPA implementation. | RPA in finance reduced the need for five full-time employees, leading to significant savings. |
Cost per Transaction Reduction | Measures the cost of executing a transaction before and after RPA. | An Icelandic healthcare institution saved over $75,000 annually by integrating automation. |
Compliance Cost Savings | Calculates savings from reduced compliance violations and penalties. | RPA ensures better adherence to regulations, saving companies thousands in compliance costs. |
Accuracy Improvement | Assesses the accuracy of outputs with and without RPA. | RPA reduced data entry errors by 90%, enhancing data accuracy. |
Process Adherence and Consistency | Evaluates adherence to standard procedures and consistency in execution. | RPA helped a Nordic municipality automate data migration, improving consistency and efficiency. |
Accupath incorporates similar principles into its manufacturing processes. By automating critical steps, the company ensures consistent quality while reducing production costs. This approach allows healthcare providers to access high-quality devices, such as the Vertebral Balloon Catheter, at competitive prices.
Time efficiency is another critical advantage of integrated OEM solutions. Streamlined workflows and advanced testing systems accelerate the development cycle. For instance, Accupath employs balloon catheter leak test systems to quickly identify defects and ensure product reliability. This reduces delays and enables faster delivery of medical devices to the market.
Customization further enhances efficiency by eliminating the need for extensive modifications during production. Accupath’s ability to tailor balloon diameters and lengths to specific medical needs minimizes waste and optimizes resource utilization. This ensures that healthcare providers receive devices that meet their exact requirements without unnecessary delays.
Tip: Partnering with experienced OEMs like Accupath not only saves costs but also ensures timely access to innovative medical devices. This combination of affordability and speed empowers healthcare providers to deliver better patient care.
Applications in the Medical Field
Integrated balloon and catheter OEM solutions have revolutionized various medical procedures, offering precision and reliability. These solutions address specific clinical challenges, enabling healthcare providers to deliver effective treatments.
Cardiovascular Procedures
Cardiovascular procedures often rely on advanced balloon and catheter systems to treat conditions like arterial blockages and heart valve disorders. Devices such as dilation balloons play a critical role in angioplasty, where they expand narrowed blood vessels to restore blood flow. Teleflex, a leader in medical device manufacturing, provides integrated solutions that ensure high performance and safety during these procedures. Their expertise in catheter design enhances trackability and pushability, essential for navigating complex vascular pathways.
Accupath's innovative approach exemplifies the benefits of customization in cardiovascular applications. By tailoring balloon diameters and lengths, their solutions meet the unique needs of patients. This customization improves procedural outcomes and reduces complications. Additionally, the integration of leak testing systems ensures the reliability of these devices under high-pressure conditions, a critical factor in cardiovascular treatments.
Gastrointestinal Treatments
In gastrointestinal treatments, balloon and catheter systems address conditions such as strictures, blockages, and bleeding. Dilation balloons are commonly used to widen narrowed sections of the esophagus or intestines, improving patient comfort and digestion. Teleflex's advanced catheter systems enhance the precision of these procedures, ensuring accurate placement and controlled inflation.
Drug-delivery balloons represent another innovation in gastrointestinal care. These devices release medication directly to affected areas, reducing systemic side effects. Accupath's commitment to quality and customization ensures that their solutions meet the specific demands of gastrointestinal treatments. Their use of high-performance materials, such as nylon and polyurethane, enhances the durability and biocompatibility of these devices.
Urological Applications
Urological applications benefit significantly from integrated balloon and catheter solutions. Therapeutic catheters address conditions like urinary blockages and kidney stones, providing effective relief for patients. AccuPath's expertise in catheter manufacturing ensures that these devices offer superior flexibility and durability, essential for navigating the urinary tract.
Specialty catheters, such as those used in prostate treatments, highlight the importance of customization. Accupath's solutions, designed with input from healthcare professionals, address the unique challenges of urological procedures. Their rigorous leak testing protocols guarantee the safety and reliability of these devices, enhancing patient outcomes.
The table below summarizes the key functionalities and applications of integrated solutions in medical procedures:
Key Functionality/Application | Description |
|---|---|
Data Sync Engine | Acts as a hub for data normalization and sharing between medical devices and healthcare systems. |
Cloud-Native Architecture | Provides scalability, flexibility, and cost-effectiveness for integration. |
Regular Assessments | Ensures ongoing performance evaluation and necessary adjustments for integration. |
Interoperability | Facilitates seamless data exchange between different systems and devices. |
Analytics | Leverages data for insightful decision-making and improved patient care. |
Continuous Improvement | Focuses on ongoing feedback and adjustments to enhance integration effectiveness. |
Accupath's dedication to innovation and quality ensures that their balloon and catheter solutions meet the evolving needs of modern healthcare. By leveraging advanced technologies and customization capabilities, they continue to lead the way in improving patient care across various medical fields.
Neurological Interventions
Neurological procedures often require precision tools to navigate delicate brain and spinal structures. Integrated balloon and catheter systems play a vital role in these interventions. They assist in treating conditions such as aneurysms, arteriovenous malformations, and spinal cord injuries. These devices enable surgeons to perform minimally invasive procedures with greater accuracy and safety.
Teleflex has contributed significantly to the development of advanced catheter systems for neurological applications. Their designs prioritize flexibility and trackability, essential for navigating complex vascular pathways in the brain. For example, diagnostic catheters help identify abnormalities, while therapeutic catheters deliver treatments directly to affected areas. These innovations improve patient outcomes by reducing the risks associated with traditional open surgeries.
Accupath's expertise in balloon and catheter OEM solutions further enhances neurological care. Their custom-engineered devices, such as the Vertebral Balloon Catheter, demonstrate the potential of integrated solutions in addressing specific medical challenges. By combining high-pressure resistance with customizable dimensions, these devices meet the unique demands of neurological procedures.
Emerging Applications in Minimally Invasive Surgery
Minimally invasive surgery continues to evolve, offering patients less pain, shorter recovery times, and reduced risks. Integrated balloon and catheter systems have become indispensable in this field. They support procedures across various specialties, including orthopedics, cardiology, and gastroenterology. These devices enable precise interventions, such as tissue dilation, drug delivery, and occlusion.
Teleflex remains at the forefront of innovation in minimally invasive surgery. Their advanced catheter systems incorporate cutting-edge materials and designs, ensuring reliability and performance. For instance, their leak testing protocols guarantee the safety of devices under high-pressure conditions. This attention to detail enhances the effectiveness of minimally invasive procedures.
Accupath's commitment to quality and customization positions them as a leader in this space. Their Vertebral Balloon Catheter exemplifies the benefits of integrated solutions in minimally invasive surgery. Designed for vertebroplasty and kyphoplasty, this device restores spinal stability with minimal disruption to surrounding tissues. Its robust construction and customizable features make it a reliable choice for healthcare providers.
Example: Vertebral Balloon Catheter by Accupath
The Vertebral Balloon Catheter by Accupath represents a breakthrough in spinal surgery. This specialized device supports vertebroplasty and kyphoplasty procedures, which treat vertebral compression fractures. Its innovative design includes a high-pressure-resistant balloon, a dual-lumen catheter, and a radiopaque ring for precise placement. These features ensure safety and effectiveness during surgery.
One of the standout aspects of this catheter is its customization. Accupath offers balloon diameters ranging from 6 to 17 millimeters and lengths from 8 to 22 millimeters. This flexibility allows surgeons to tailor the device to individual patient needs. The catheter's ability to withstand pressures up to 700 pounds further enhances its reliability.
Accupath's rigorous manufacturing processes, including advanced leak testing, ensure the quality of their devices. By adhering to international standards, they deliver products that meet the highest safety and performance requirements. The Vertebral Balloon Catheter exemplifies Accupath's dedication to innovation and excellence in balloon and catheter OEM solutions.
Note: Accupath's integrated solutions empower healthcare providers to deliver better patient care. Their commitment to customization and quality sets them apart in the medical device industry.
Choosing the Right OEM Partner
Selecting the right OEM partner is a critical step in ensuring the success of integrated balloon and catheter solutions. A reliable partner brings expertise, regulatory knowledge, and customization capabilities to the table, enabling healthcare providers to deliver high-quality medical devices.
Factors to Consider
Expertise and Experience
An OEM partner's expertise and experience directly impact the quality and reliability of the final product. Companies with a proven track record in balloon and catheter manufacturing demonstrate their ability to handle complex projects. Their experience ensures that they understand the nuances of medical device production, from material selection to advanced testing methods. For instance, Accupath leverages its extensive expertise to deliver innovative solutions like the Vertebral Balloon Catheter, which meets the rigorous demands of spinal surgeries.
Regulatory Knowledge
Navigating the regulatory landscape is essential for bringing medical devices to market. An ideal OEM partner should possess in-depth knowledge of global standards, such as FDA and ISO certifications. This ensures that the devices comply with safety and performance requirements. Accupath exemplifies this by adhering to stringent quality assurance protocols, including leak testing, to guarantee the reliability of its products. Their commitment to regulatory compliance minimizes risks and accelerates time-to-market for healthcare providers.
Customization Capabilities
Customization is a key factor when addressing specific medical needs. An OEM partner should offer the flexibility to tailor products to unique clinical applications. Accupath excels in this area by providing customizable balloon diameters and lengths for its Vertebral Balloon Catheter. This adaptability allows healthcare professionals to deliver precise and effective treatments, enhancing patient outcomes.
Questions to Ask Potential OEM Partners
When evaluating OEM partners, asking the right questions can help identify the best fit for your needs. Consider the following:
What is your experience in manufacturing balloon and catheter systems for medical applications?
How do you ensure compliance with regulatory standards?
Can you provide examples of customized solutions you have developed?
What testing protocols, such as leak testing, do you use to validate product reliability?
How do you handle challenges during the development process?
These questions provide valuable insights into the partner's capabilities and commitment to quality. By choosing a partner like Accupath, healthcare providers gain access to innovative, reliable, and customizable solutions that meet the evolving demands of the medical industry.
Integrated balloon and catheter OEM solutions have transformed the medical field by offering precision, reliability, and customization. These innovations address specific healthcare challenges, enabling providers to deliver better patient outcomes. By streamlining manufacturing and ensuring regulatory compliance, OEM solutions drive advancements in medical technology. Companies like Accupath exemplify this progress through their commitment to quality and innovation. Exploring OEM partnerships allows healthcare providers to access cutting-edge devices tailored to their needs. This collaboration fosters growth and ensures that patients benefit from the latest advancements in care.
FAQ
What are integrated balloon and catheter OEM solutions?
Integrated balloon and catheter OEM solutions involve the design and manufacturing of devices that combine balloons and catheters for specific medical applications. These solutions ensure precision, reliability, and customization to meet healthcare needs.
Why is customization important in balloon and catheter solutions?
Customization allows manufacturers to tailor devices to specific patient needs. This improves treatment precision and enhances patient outcomes, especially in procedures like spinal surgeries or cardiovascular interventions.
How do manufacturers ensure the safety of balloon catheters?
Manufacturers use advanced testing methods, such as leak testing, to detect defects and validate device performance. These tests ensure that balloon catheters meet stringent safety and reliability standards.
What materials are commonly used in balloon and catheter systems?
Materials like nylon, polyester, polyurethane, and polyethylene are commonly used. These materials provide durability, flexibility, and biocompatibility, ensuring the devices perform effectively during medical procedures.
How do integrated solutions benefit healthcare providers?
Integrated solutions streamline manufacturing, reduce costs, and improve device reliability. They enable healthcare providers to access high-quality, customizable devices that enhance patient care and procedural efficiency.
What makes Accupath’s Vertebral Balloon Catheter unique?
Accupath’s Vertebral Balloon Catheter offers high-pressure resistance, customizable dimensions, and advanced leak testing. These features ensure safety and reliability during spinal surgeries, making it a trusted choice for healthcare professionals.
How do OEM solutions contribute to innovation in the medical field?
OEM solutions foster innovation by enabling the development of advanced, precision-engineered devices. They support healthcare providers in delivering better patient outcomes through cutting-edge technology.
Why should healthcare providers choose Accupath as their OEM partner?
Accupath combines expertise, regulatory compliance, and customization capabilities. Their commitment to quality ensures that healthcare providers receive reliable, high-performance devices tailored to their needs.
See Also
Three Essential Steps for Selecting Top PKP Balloon Services
Comprehensive Guide to Selecting the Perfect PKP Balloon Catheter
Detailed Instructions for Effective Microcatheter Production

