Etched PTFE for Catheter Manufacturing: What’s New?

Etched PTFE for catheter manufacturing has transformed the industry by introducing groundbreaking advancements. Recent innovations in etching techniques have enhanced the bonding capabilities of PTFE, making it more adaptable for complex medical applications. Manufacturers now achieve ultra-thin liners with exceptional coating consistency, optimizing catheter performance and reducing friction during minimally invasive procedures. Automation in production processes has further improved precision and efficiency, ensuring consistent quality. These advancements not only elevate product reliability but also streamline compliance with stringent regulatory standards, setting a new benchmark for excellence in the medical device industry.
Key Takeaways
Etched PTFE enhances bonding capabilities, making it essential for reliable catheter manufacturing in complex medical applications.
Recent innovations in etching techniques allow for ultra-thin liners with consistent coating thickness, optimizing catheter performance and reducing friction.
Automation in production processes improves precision and efficiency, ensuring consistent quality and compliance with regulatory standards.
The biocompatibility of etched PTFE minimizes the risk of adverse reactions, ensuring patient safety during catheter use.
Sustainability efforts in etched PTFE manufacturing focus on environmentally friendly processes and recycling initiatives, promoting a greener future.
Future trends include the integration of smart technologies in catheters, enabling real-time data collection and enhancing patient care.
Overview of Etched PTFE for Catheter Manufacturing
What is Etched PTFE?
Etched PTFE refers to polytetrafluoroethylene that has undergone a surface modification process to enhance its bonding capabilities. PTFE, known for its non-stick and lubricious properties, typically resists adhesion. The etching process chemically alters the surface, enabling it to bond effectively with other materials. This transformation makes etched PTFE an essential component in medical applications, particularly in catheter manufacturing.
The etching process involves treating the PTFE surface with specialized chemicals. This treatment modifies the molecular structure, creating a surface that adheres well to adhesives and other components. Manufacturers often use etched PTFE in catheter liners due to its ability to maintain a smooth, low-friction surface while offering enhanced adhesion. These properties ensure that catheters perform reliably in demanding medical environments.
Why Etched PTFE is Critical for Catheters
Etched PTFE plays a pivotal role in catheter manufacturing by addressing the unique challenges of medical device assembly. Its enhanced bonding properties allow it to integrate seamlessly with other materials, such as hubs and connectors. This integration ensures the structural integrity of the catheter, which is vital for its performance during medical procedures.
The material's biocompatibility further underscores its importance. PTFE minimizes the risk of allergic reactions or adverse responses in patients, making it a safe choice for medical applications. Additionally, its low-friction surface reduces the risk of tissue damage and infection, ensuring patient safety during catheter insertion and use.
In catheter manufacturing, etched PTFE also contributes to precision and efficiency. The ability to produce ultra-thin liners with consistent coating thickness optimizes the catheter's lumen, facilitating smoother delivery of medical devices. This consistency enhances the overall functionality of the catheter, particularly in minimally invasive procedures where precision is critical.
"Etched PTFE tubing offers a solution for bonding PTFE with other materials by altering its surface properties through dip etching." This capability highlights its versatility and adaptability in various catheter designs, from introducers to dilators.
By combining durability, biocompatibility, and enhanced adhesion, etched PTFE has become indispensable in modern catheter manufacturing. Its unique properties not only improve the performance of medical devices but also set new standards for quality and reliability in the healthcare industry.
Latest Advancements in Etched PTFE for Catheter Manufacturing
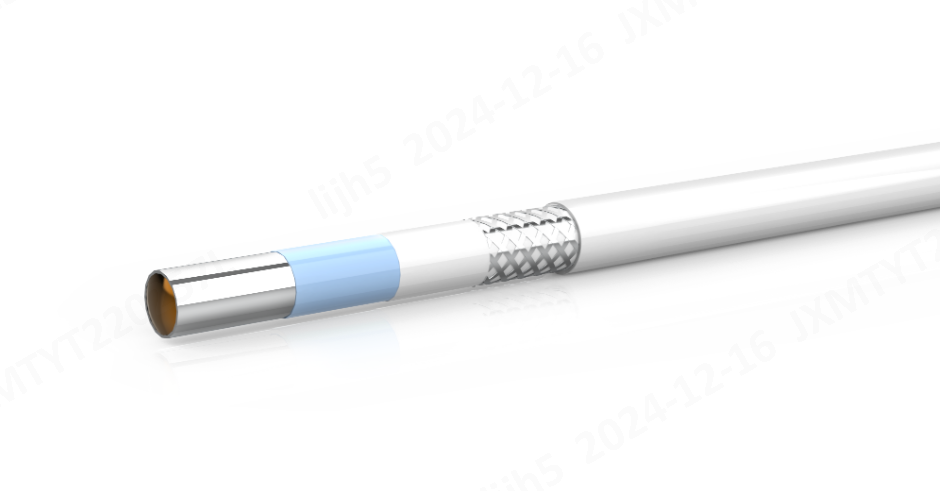
Innovations in Etching Techniques
Recent advancements in etching techniques have significantly improved the manufacturing process of etched PTFE for catheter manufacturing. The chemical surface treatment now employs more precise methods to modify the molecular structure of PTFE. These innovations enhance its adhesive properties, overcoming the material's natural resistance to bonding. Manufacturers have adopted advanced etching solutions that ensure uniform surface modification, which is critical for consistent performance in catheter applications.
The introduction of environmentally friendly etching processes has also gained traction. These methods reduce the use of hazardous chemicals while maintaining the effectiveness of the surface treatment. By refining the etching process, manufacturers achieve better adhesion between PTFE and other catheter components, such as connectors and hubs. This improvement ensures the structural integrity of the final product, even under demanding medical conditions.
"Etching is a surface treatment process that involves chemically modifying the surface of PTFE to enhance its adhesive properties or improve its wettability." This process remains essential for integrating PTFE into catheter designs, ensuring reliable performance during medical procedures.
Enhanced Material Properties
The material properties of etched PTFE have seen remarkable enhancements, making it more suitable for modern catheter manufacturing. Ultra-thin PTFE liners with exceptional coating thickness consistency are now achievable. These liners maximize the catheter lumen, allowing for smoother delivery of medical devices during minimally invasive procedures. The improved consistency in material thickness reduces variability, ensuring predictable and reliable catheter performance.
Etched PTFE now exhibits greater durability and flexibility, which are crucial for medical applications. The material's low coefficient of friction minimizes resistance during catheter insertion, reducing the risk of tissue damage. Additionally, its biocompatibility ensures patient safety by minimizing adverse reactions. These enhanced properties make etched PTFE indispensable for creating high-performance catheters that meet the rigorous demands of the healthcare industry.
Automation and Precision Manufacturing
Automation has revolutionized the production of etched PTFE for catheter manufacturing. Advanced machinery and robotics now handle critical steps in the manufacturing process, ensuring precision and efficiency. Automated systems maintain consistent quality by eliminating human error, which is vital for meeting stringent regulatory standards in the medical device industry.
Precision manufacturing techniques have also improved the integration of etched PTFE into catheter designs. Computer-controlled processes allow for exact measurements and alignments, ensuring that each component fits seamlessly. This level of precision enhances the overall functionality of the catheter, particularly in applications requiring high accuracy, such as cardiology and vascular interventions.
The combination of automation and precision manufacturing has streamlined production workflows. Manufacturers can now produce etched PTFE liners at a faster rate without compromising quality. This efficiency reduces production costs and accelerates the delivery of medical devices to the market, benefiting both manufacturers and healthcare providers.
Benefits of Advancements in Etched PTFE for Catheter Manufacturing
Improved Product Quality
Advancements in etched PTFE technology have significantly elevated the quality of catheters. The ability to produce ultra-thin liners with consistent coating thickness ensures uniformity in catheter performance. This consistency minimizes variability, which is critical for medical devices used in high-stakes procedures. Enhanced surface properties, such as improved lubricity and biocompatibility, further contribute to the reliability of catheters. These features reduce friction during insertion, lowering the risk of tissue damage and ensuring patient safety.
The precise control over surface roughness achieved through modern etching techniques also enhances bonding capabilities. This improvement allows manufacturers to create catheters with superior structural integrity. For example, etched PTFE liners now accommodate wall thicknesses as low as 0.001 inches, enabling the production of highly efficient and compact designs. These advancements set a new standard for product quality in the medical device industry.
"Etched PTFE tubing offers exceptional performance by combining durability, flexibility, and enhanced adhesion," making it indispensable for high-performance catheter manufacturing.
Enhanced Manufacturing Efficiency
The integration of advanced manufacturing methods has streamlined the production of etched PTFE for catheter manufacturing. Automation plays a pivotal role in this transformation. Robotic systems and computer-controlled processes ensure precision at every stage, from etching to assembly. This level of accuracy reduces errors, leading to fewer defective products and less material waste.
Modern etched PTFE liners also simplify the manufacturing process. Their improved bonding properties eliminate the need for additional adhesives or complex assembly steps. For instance, dipped etched lengths ranging from 0.050 to 5 inches allow for seamless integration into various catheter designs. These efficiencies accelerate production timelines, enabling manufacturers to meet growing market demands without compromising quality.
The adoption of environmentally friendly etching processes further enhances efficiency. By reducing the reliance on hazardous chemicals, manufacturers not only improve workplace safety but also lower operational costs. These advancements collectively optimize manufacturing workflows, benefiting both producers and end-users.
Meeting Regulatory Standards
The medical device industry operates under stringent regulatory requirements, and advancements in etched PTFE technology help manufacturers achieve compliance more effectively. Consistent coating thickness and precise material properties ensure that catheters meet the rigorous standards set by regulatory bodies. These improvements reduce the likelihood of product recalls or failures, safeguarding both patients and manufacturers.
Enhanced biocompatibility is another critical factor. Etched PTFE minimizes the risk of adverse reactions, making it a preferred choice for medical applications. The ability to produce liners with sub-70° contact angles further demonstrates the material's suitability for demanding environments. These properties align with regulatory expectations for safety and performance.
Automation also supports compliance efforts. By eliminating human error, automated systems ensure that every product meets the required specifications. This consistency simplifies the documentation and validation processes, which are essential for regulatory approval. Manufacturers leveraging these advancements position themselves as leaders in the competitive medical device market.
Future Trends in Etched PTFE for Catheter Manufacturing
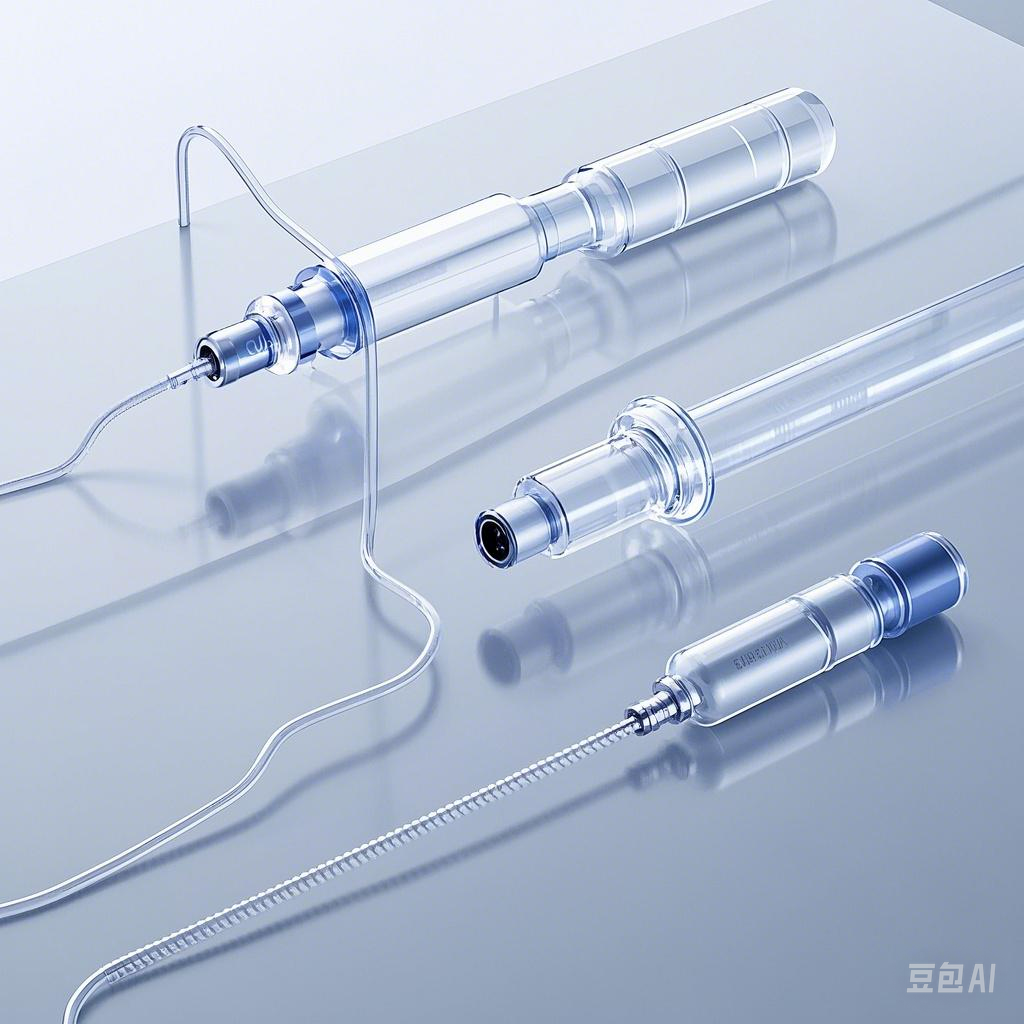
Material Innovations
The future of catheter manufacturing relies heavily on advancements in material science. Etched PTFE continues to evolve, with researchers focusing on enhancing its properties to meet the growing demands of medical applications. One significant trend involves the development of ultra-thin PTFE liners with improved coating uniformity. These liners maximize the catheter lumen, enabling smoother device delivery and reducing patient discomfort during minimally invasive procedures.
Manufacturers are also exploring ways to improve the durability and flexibility of etched PTFE. Enhanced formulations aim to withstand extreme medical environments while maintaining the material's low-friction surface. This ensures reliable performance even in high-pressure applications, such as cardiology and vascular interventions. Additionally, innovations in dip etching techniques allow for more precise surface modifications, further improving the bonding capabilities of PTFE with other catheter components.
"Etching alters the surface properties of PTFE, making it more amenable to wetting by liquids or coatings." This advancement is particularly valuable in applications where the liner must interact with or contain liquids, ensuring optimal functionality.
Integration of Smart Technologies
The integration of smart technologies into catheter manufacturing represents a transformative trend. Automated systems equipped with artificial intelligence (AI) and machine learning (ML) algorithms now monitor and optimize the etching process. These technologies ensure consistent surface treatment, reducing variability and enhancing product quality. AI-driven analytics also provide real-time feedback, enabling manufacturers to identify and address potential issues before they impact production.
Smart sensors embedded within manufacturing equipment further enhance precision. These sensors measure critical parameters, such as coating thickness and surface roughness, ensuring that each etched PTFE liner meets exact specifications. This level of control not only improves the reliability of catheters but also accelerates production timelines, meeting the increasing demand for high-performance medical devices.
The concept of "smart catheters" is also gaining traction. By integrating etched PTFE with advanced electronics, manufacturers can create catheters capable of real-time data collection and transmission. These devices could revolutionize patient care by providing healthcare professionals with immediate insights during procedures.
Sustainability Efforts
Sustainability has become a key focus in the medical device industry, and etched PTFE manufacturing is no exception. Companies are adopting environmentally friendly etching processes to minimize the use of hazardous chemicals. These methods not only reduce environmental impact but also improve workplace safety, aligning with global sustainability goals.
Recycling initiatives are another area of interest. Researchers are investigating ways to repurpose etched PTFE waste, transforming it into usable materials for other applications. This approach reduces material waste and promotes a circular economy within the industry.
Energy-efficient manufacturing practices are also gaining momentum. Advanced machinery designed to consume less energy during the etching process helps lower the carbon footprint of catheter production. By prioritizing sustainability, manufacturers can meet regulatory requirements while contributing to a greener future.
At AccuPath Group Co., Ltd., the focus on precision and excellence extends to sustainability efforts, ensuring that high-quality solutions align with environmental responsibility.
The advancements in etched PTFE for catheter manufacturing have redefined industry standards. Enhanced etching techniques, improved material properties, and automation have collectively elevated product quality and manufacturing efficiency. These innovations ensure catheters meet stringent regulatory requirements while delivering superior clinical outcomes. By reducing friction and improving adhesion, etched PTFE optimizes catheter performance, minimizing risks like tissue damage and infection. As the medical field evolves, staying ahead of trends such as material innovations and sustainability will remain essential for meeting future demands and maintaining excellence in catheter manufacturing.
FAQ
What is the manufacturing process of PTFE-lined catheters?
The manufacturing process of PTFE-lined catheters involves several precise steps to ensure optimal performance. PTFE liners undergo an etching process to prepare their surface for bonding with other materials like metals, polymers, or elastomers. This chemical surface treatment modifies the PTFE, enabling a stronger and more durable bond. After etching, manufacturers integrate the PTFE liners into catheter designs, ensuring smooth delivery and reliable performance in medical applications.
Why is etched PTFE preferred for catheter manufacturing?
Etched PTFE is preferred due to its unique combination of properties. The etching process enhances PTFE's adhesive capabilities, allowing it to bond seamlessly with other materials. Its low-friction surface minimizes resistance during insertion, reducing the risk of tissue damage. Additionally, PTFE's biocompatibility ensures patient safety, making it an ideal choice for medical devices used in sensitive environments.
How does automation improve the production of etched PTFE?
Automation enhances the production of etched PTFE by increasing precision and efficiency. Advanced machinery and robotics handle critical steps, such as etching and assembly, with minimal errors. Automated systems ensure consistent quality, which is essential for meeting regulatory standards. This streamlined process reduces production costs and accelerates the delivery of high-quality medical devices to the market.
What are the benefits of ultra-thin PTFE liners in catheters?
Ultra-thin PTFE liners offer several advantages in catheter manufacturing. These liners maximize the catheter lumen, facilitating smoother delivery of medical devices during minimally invasive procedures. Their consistent coating thickness ensures uniform performance, reducing variability and enhancing reliability. The thin design also allows for compact and efficient catheter structures, meeting the demands of modern medical applications.
Are there environmentally friendly etching processes available?
Yes, environmentally friendly etching processes have been developed to reduce the use of hazardous chemicals. These methods maintain the effectiveness of surface treatment while minimizing environmental impact. By adopting these sustainable practices, manufacturers improve workplace safety and align with global efforts to reduce ecological footprints.
How does etched PTFE contribute to regulatory compliance?
Etched PTFE contributes to regulatory compliance by ensuring consistent material properties and performance. The precise control over coating thickness and surface roughness meets the stringent standards set by regulatory bodies. Enhanced biocompatibility further supports compliance, as it minimizes the risk of adverse reactions in patients. Automation in manufacturing also ensures that each product adheres to required specifications, simplifying validation and approval processes.
What role does etched PTFE play in minimally invasive procedures?
Etched PTFE plays a crucial role in minimally invasive procedures by enhancing catheter performance. Its low-friction surface reduces resistance during insertion, minimizing tissue damage and patient discomfort. The material's durability and flexibility ensure reliable operation in demanding medical environments. These properties make etched PTFE indispensable for procedures requiring precision and efficiency.
Can etched PTFE be used in smart catheter designs?
Yes, etched PTFE can be integrated into smart catheter designs. Its ability to bond with advanced electronics enables the creation of catheters capable of real-time data collection and transmission. These smart devices provide healthcare professionals with immediate insights during procedures, improving patient outcomes and advancing medical technology.
What advancements have been made in PTFE etching techniques?
Recent advancements in PTFE etching techniques include the development of more precise and environmentally friendly processes. These innovations ensure uniform surface modification, enhancing adhesion and performance. Dip etching methods now allow for greater control over surface properties, enabling the production of ultra-thin liners with exceptional coating consistency.
How does sustainability impact etched PTFE manufacturing?
Sustainability has become a key focus in etched PTFE manufacturing. Companies are adopting energy-efficient machinery and recycling initiatives to reduce waste and lower carbon footprints. Environmentally friendly etching processes further support these efforts by minimizing the use of harmful chemicals. These practices align with industry goals to create high-quality medical devices while promoting environmental responsibility.
See Also
Exploring The Role Of PTFE Etched Liners In Catheters
The Importance Of PTFE Etched Liners In Catheter Reinforcement

