A Guide to Choosing Reliable Nitinol Tubing for Medical Applications
Selecting medical grade nitinol tubing for implants plays a pivotal role in ensuring patient safety and device performance. High-quality nitinol tubing must meet stringent standards to function reliably in medical applications. For example, heart valve frames endure over 600 million cardiac cycles, while endovascular implants withstand up to 400 million arterial pressure cycles. These demanding conditions require materials with exceptional durability and compliance with ASTM F 2063 standards, which limit nonmetallic inclusions to 39 microns in size. Meeting such benchmarks ensures medical devices perform effectively and improve patient outcomes. The growing reliance on nitinol in stents and catheters further underscores the need for precision and reliability in material selection.
Key Takeaways
Nitinol tubing can bend and return to its shape. This makes it great for medical implants that need to adjust without breaking.
It is safe for the body because its titanium coating stops harmful nickel from leaking out.
The size and thickness of nitinol tubing must be exact. This helps it fit medical devices and prevents problems.
Pick suppliers with ISO 13485 and FDA approval. These certifications mean the tubing is high quality and safe for medical use.
Special coatings on nitinol tubing make it work better. They help it last longer and stay safe for patients.
Key Material Properties of Nitinol

Superelasticity and Shape Memory
Nitinol exhibits two remarkable characteristics: superelasticity and shape memory properties. These features make it indispensable in medical applications. Superelasticity allows nitinol to undergo significant deformation and return to its original shape without requiring heat. This property is particularly useful in stents and orthodontic wires, where flexibility and resilience are critical. Shape memory properties enable nitinol to recover its pre-deformed shape when exposed to a specific temperature. This behavior is achieved through a phase transformation between austenite and martensite structures, which occurs at precise thermal thresholds.
Empirical studies highlight the impact of superelasticity on medical implants. For instance, nitinol demonstrates a strain recovery of 5.62% and a recovery ratio of 98%, ensuring reliable performance in patient-specific biomedical devices. These attributes reduce the risk of mechanical failure, even under repetitive stress cycles. The combination of superelasticity and shape memory properties enhances the adaptability of nitinol in dynamic environments, making it a preferred material for minimally invasive procedures.
Biocompatibility for Medical Applications
Biocompatibility is a cornerstone of nitinol's success in medical applications. The material's surface forms a stable titanium oxide layer, which prevents nickel ion leaching. This protective layer minimizes the risk of adverse reactions, ensuring compatibility with the human body. Clinical evidence supports nitinol's long-term biocompatibility, demonstrating its ability to maintain structural integrity and reduce complications in implants.
The titanium oxide layer also contributes to nitinol's corrosion resistance, which is vital for implants exposed to bodily fluids. This dual functionality ensures that nitinol remains safe and effective over extended periods. Its biocompatibility has led to widespread use in cardiovascular stents, orthopedic implants, and dental devices, where patient safety is paramount.
Corrosion Resistance and Longevity
Nitinol's corrosion resistance is another critical property that ensures its reliability in medical applications. The titanium oxide layer not only enhances biocompatibility but also protects the material from degradation in harsh environments. This resistance to corrosion is essential for implants that must endure prolonged exposure to bodily fluids and varying pH levels.
Studies have shown that nitinol's corrosion resistance contributes significantly to its longevity. For example, vascular implants made from nitinol maintain their structural integrity even after years of use. The material's high fatigue resistance further extends its lifespan, allowing it to withstand millions of stress cycles without failure. These properties make nitinol a durable and dependable choice for medical devices, ensuring consistent performance and improved patient outcomes.
Dimensional Precision in Medical Grade Nitinol Tubing
Precision plays a critical role in the manufacturing of medical grade nitinol tubing. Accurate dimensions ensure the tubing performs reliably in demanding applications, such as cardiovascular stents and orthopedic implants. Manufacturers must meet stringent specifications to guarantee the tubing's functionality and compatibility with medical devices.
Diameter and Wall Thickness Accuracy
Maintaining precise diameter and wall thickness is essential for nitinol tubing used in medical applications. Advanced measurement protocols validate these dimensions during production. Precision tools, including laser scanners and electronic instruments, verify uniformity in wall thickness and diameter. Techniques like laser cutting and drawing further enhance dimensional accuracy. Inspection tools, such as laser micrometers and ultrasonic gauges, ensure the tubing meets exact specifications. Non-destructive testing methods, including ultrasonic inspection, identify inconsistencies in wall thickness without compromising the material's integrity. These processes ensure nitinol tubing achieves the required accuracy for critical medical devices.
Importance of Tight Tolerances
Tight tolerances are vital for nitinol tubing to meet the rigorous demands of medical applications. Premium-grade tubing achieves tolerances as tight as ±0.0005", ensuring over 95% concentricity accuracy. This level of precision is critical for implants requiring exact specifications, such as capillary nitinol tubes. Quality control measures, including laser micrometry and ultrasonic testing, validate these tolerances. Surface finishing techniques, such as electropolishing, further refine dimensional accuracy and enhance performance characteristics like flow and corrosion resistance. Tight tolerances reduce the risk of device failure and improve patient outcomes.
Consistency in Manufacturing Standards
Consistency in manufacturing standards ensures nitinol tubing maintains its reliability across production batches. Advanced monitoring systems track parameters during the drawing process to maintain dimensional stability. Statistical studies confirm superior fatigue performance in TM-1 manufacturing techniques, which achieve a probabilistic fatigue endurance limit (FEL) 2-3 times greater than TM-2 methods. Standard-grade nitinol tubing exhibits strong dependence on inclusion size, with predictive relationships established to ensure consistent quality. These manufacturing protocols guarantee nitinol tubing meets the stringent requirements of medical applications.
Surface Quality and Coating Options
Smooth Surface Finish for Medical Devices
A smooth surface finish is essential for nitinol tubing used in medical devices. It minimizes friction and reduces the risk of tissue damage during implantation or use. Polishing and chemical treatments enhance the tubing's surface, making it safer and more resistant to corrosion. These processes ensure the tubing performs reliably in demanding environments, such as cardiovascular systems and orthopedic applications.
Smooth surfaces also play a critical role in reducing complications associated with implants and surgical instruments. For example, a polished nitinol surface lowers the likelihood of bacterial adhesion, improving patient outcomes. The finishing process involves advanced techniques like electropolishing and passivation, which refine the tubing's exterior while maintaining its structural integrity.
Source | Evidence |
|---|---|
Nitinol Tubing Grades | A smooth surface finish is also important. These features make devices work well in tough conditions. |
Medical Device Finishing Solutions | Smooth surfaces on medical devices, such as implants and surgical instruments, minimize the risk of complications, making the finishing process a critical step in medical manufacturing. |
How Nitinol Tubing is Manufactured | Surface finish shows how smooth the outside of the tubing is. A smooth surface lowers friction and reduces harm to body tissues. Polishing and chemical treatments make the tubing safer and more resistant to rust. |
Coating Options for Enhanced Performance
Coating options further enhance the performance of nitinol tubing in medical applications. Specialized coatings improve biocompatibility, reduce wear, and increase corrosion resistance. For example, hydrophilic coatings create a lubricious surface, facilitating smoother device insertion and reducing patient discomfort. Antimicrobial coatings prevent bacterial growth, ensuring the tubing remains sterile during use.
Manufacturers often apply coatings tailored to specific medical applications. Cardiovascular stents benefit from drug-eluting coatings that release therapeutic agents over time. Orthopedic implants utilize wear-resistant coatings to extend their lifespan. These advancements in coating technology optimize nitinol tubing for diverse medical needs, ensuring its reliability and effectiveness.
Avoiding Defects and Contaminants
Defects and contaminants compromise the performance and safety of nitinol tubing. Manufacturers implement stringent quality control measures to detect and eliminate imperfections during production. Non-destructive testing methods, such as ultrasonic inspection, identify surface irregularities without damaging the material.
Contaminants, including residual oils and debris, are removed through cleaning processes like ultrasonic cleaning and acid etching. These steps ensure the tubing meets the high standards required for medical applications. A defect-free nitinol surface reduces the risk of device failure and enhances patient safety, making it a critical aspect of manufacturing.
Tip: Partnering with suppliers who prioritize defect-free production ensures consistent quality and compliance with medical standards.
Application-Specific Requirements
Customization for Implant Designs
Medical implants often require tailored designs to meet specific functional and anatomical needs. Nitinol's unique properties, such as its ability to undergo a diffusionless phase transformation between austenite and martensite, make it ideal for customization. This transformation enables superelasticity, which is essential for implants like stents. These devices must be crimped for insertion and then expanded without permanent deformation. The microstructural complexity of nitinol, including variations in martensite and austenite domains, highlights the importance of application-specific customization. Tailored approaches optimize the material's performance, ensuring it meets the demands of diverse medical applications.
For example, cardiovascular stents benefit from nitinol's ability to conform to dynamic environments. Orthopedic implants, on the other hand, require precise adjustments to accommodate varying load conditions. Manufacturers collaborate with medical professionals to design nitinol tubing that aligns with the unique requirements of each implant type. This level of customization enhances device functionality and improves patient outcomes.
Compatibility with Regulatory Standards
Regulatory compliance is critical for medical-grade nitinol tubing. Organizations such as the FDA and ISO establish stringent standards to ensure the safety and efficacy of medical devices. Nitinol tubing must meet these requirements to gain approval for use in implants. For instance, ISO 13485 certification ensures that manufacturing processes adhere to quality management systems specific to medical devices. ASTM F2063 outlines the material properties and testing protocols for nitinol used in biomedical applications.
Manufacturers must document every step of the production process to demonstrate compliance. This includes material sourcing, dimensional accuracy, and surface quality. Regular audits and inspections verify adherence to regulatory standards. By meeting these requirements, nitinol tubing ensures patient safety and maintains the integrity of medical devices.
Meeting Medical Device Performance Needs
Medical devices must perform reliably under challenging conditions. Nitinol tubing excels in this regard due to its exceptional durability and adaptability. Its superelasticity allows it to withstand repetitive stress cycles without permanent deformation. This property is particularly valuable in cardiovascular and endovascular implants, which endure millions of cycles during their lifespan.
Additionally, nitinol's corrosion resistance ensures longevity in environments exposed to bodily fluids. Manufacturers optimize the tubing's properties to meet specific performance needs, such as flexibility, fatigue resistance, and biocompatibility. Advanced testing protocols validate these characteristics, ensuring the tubing performs as intended. By addressing performance requirements, nitinol tubing supports the development of innovative medical devices that improve patient care.
Selecting a Reliable Supplier for Nitinol Tubing
Choosing the right supplier for nitinol tubing is a critical step in ensuring the success of medical devices. A reliable supplier not only guarantees high-quality materials but also provides the expertise and compliance necessary to meet stringent medical standards. Below are the key factors to consider when selecting a supplier.
Certifications and Compliance (e.g., ISO 13485, FDA)
Certifications such as ISO 13485 and FDA clearance are essential indicators of a supplier's commitment to quality and regulatory compliance. These certifications demonstrate that the supplier adheres to rigorous quality management systems tailored to medical devices. For instance, ISO 13485 ensures that the manufacturing processes meet international standards, while FDA clearance validates the safety and efficacy of the nitinol tubing for use in medical applications.
Suppliers with these certifications offer several advantages. They enhance customer trust by demonstrating adherence to global quality standards. Their compliance with regulations often leads to improved operational efficiency and reduced waste. Additionally, certified suppliers can identify and resolve potential risks early, ensuring better decision-making and fewer complications during production. Meeting these standards also opens access to new markets, providing opportunities for growth and increased competitiveness.
Key Points | Description |
|---|---|
Customer Trust | Certification demonstrates adherence to quality standards, enhancing customer confidence in product safety. |
Better Processes | Compliance with regulations leads to improved operational efficiency and reduced waste. |
Risk Control | Early identification and resolution of issues contribute to better decision-making and fewer complications. |
Market Growth | Meeting compliance opens access to new markets, increasing sales opportunities. |
Global Standards | Adhering to international regulations enhances reputation and competitiveness. |
Manufacturing Expertise and Capabilities
A supplier's manufacturing expertise plays a pivotal role in delivering high-quality nitinol tubing. Advanced manufacturing techniques, such as laser cutting and precision drawing, ensure that the tubing meets the exacting requirements of medical applications. Suppliers with vertically integrated operations, like Resonetics, provide distinct advantages. By managing every stage of production—from the nitinol melt to final processing—they ensure consistency, quality control, and efficiency.
The nitinol tubing market is dominated by key players like Fort Wayne Metals, Confluent Medical Technologies, and SAES Group, which collectively hold over 65% of the global market share. These companies leverage specialized capabilities to address high-growth applications in medical devices such as stents and guidewires. Their focus on innovation and precision manufacturing sets a benchmark for the industry.
A vertically integrated supplier offers unmatched consistency and material understanding. By overseeing every stage of production, they provide immediate and long-term feedback to the supply chain, ensuring superior quality.
Supplier Name | Key Attributes |
|---|---|
Cadence, Inc. | Dedication to quality ensures reliable and high-performing nitinol. |
Confluent Medical Technologies | Emphasizes product quality with advanced manufacturing techniques and stringent quality control. |
Fort Wayne Metals | Dominates the market with a strong focus on high-growth applications in medical devices. |
Quality Assurance and Testing Protocols
Quality assurance is a cornerstone of reliable nitinol tubing production. Suppliers must implement stringent testing protocols to ensure the tubing meets the high standards required for medical applications. Non-destructive testing (NDT) methods, such as ultrasonic and eddy current testing, detect internal flaws and surface defects without compromising the material's integrity. Dimensional inspections verify the tubing's outer diameter, wall thickness, and concentricity, ensuring precise specifications. Mechanical inspections assess strength, elasticity, and fatigue resistance, confirming the tubing's performance under stress.
Suppliers that adhere to industry standards, such as ASTM and ISO benchmarks, provide an added layer of reliability. These standards define the material properties and testing protocols necessary for medical-grade nitinol. Advanced quality assurance practices not only ensure compliance but also enhance the tubing's durability and performance, making it suitable for demanding medical applications.
Testing Protocols | Description |
|---|---|
Non-Destructive Testing (NDT) | Techniques like ultrasonic and eddy current testing to detect internal flaws and surface defects. |
Dimensional Inspections | Measurement of outer diameter, wall thickness, and concentricity to ensure precise specifications. |
Mechanical Inspections | Tests for strength, elasticity, and fatigue resistance to confirm performance under stress. |
Compliance with Industry Standards | Adherence to ASTM and ISO benchmarks for material properties and testing protocols. |
Tip: Partnering with a supplier that prioritizes robust quality assurance practices ensures consistent performance and compliance with medical standards.
By selecting a supplier with certifications, advanced manufacturing capabilities, and rigorous quality assurance protocols, medical device manufacturers can secure high-quality nitinol tubing that meets the demands of modern healthcare applications.
Choosing the right nitinol tubing for medical implants involves evaluating several critical factors. Material properties like superelasticity, biocompatibility, and corrosion resistance ensure the tubing performs reliably under demanding conditions. Dimensional precision, including tight tolerances and consistent manufacturing standards, guarantees compatibility with medical devices. Surface quality and advanced coatings further enhance performance and safety.
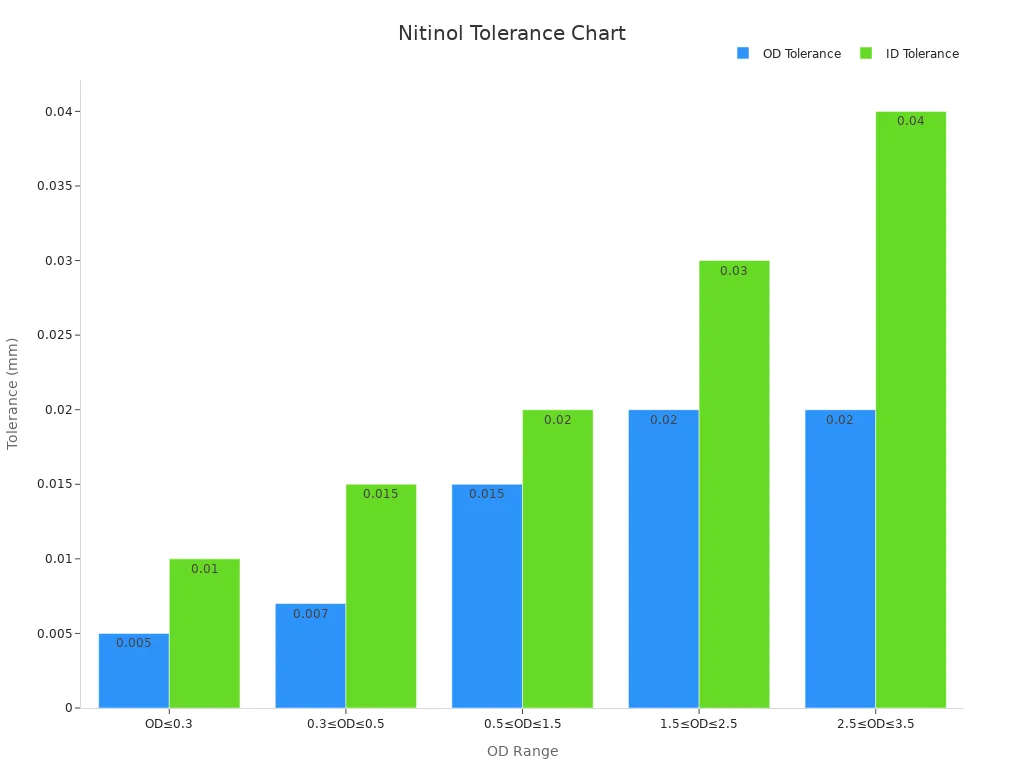
Actionable steps include prioritizing suppliers with certifications like ISO 13485 and FDA compliance. These certifications ensure adherence to stringent quality standards. Manufacturers should also assess a supplier's expertise in advanced manufacturing techniques and robust quality assurance protocols. For example, the following table highlights the importance of dimensional accuracy in nitinol tubing:
Outer Diameter (OD) (mm) | OD Tolerance (mm) | ID Tolerance (mm) |
|---|---|---|
OD≤0.3 | ±0.005 | ±0.010 |
0.3≤OD≤0.5 | ±0.007 | ±0.015 |
0.5≤OD≤1.5 | ±0.015 | ±0.020 |
1.5≤OD≤2.5 | ±0.020 | ±0.030 |
2.5≤OD≤3.5 | ±0.020 | ±0.040 |
Additionally, nitinol tubing has demonstrated minimal tissue reactions post-implantation and sustained reductions in intraocular pressure over six months. These outcomes underscore its reliability and safety in medical applications. The chart below illustrates the relationship between outer diameter ranges and tolerances:
Partnering with a certified and experienced supplier ensures access to high-quality nitinol tubing that meets regulatory standards and supports innovative medical device development. This collaboration ultimately enhances patient outcomes and advances healthcare solutions.
FAQ
What makes nitinol tubing suitable for medical implants?
Nitinol tubing offers superelasticity, shape memory, and biocompatibility. These properties allow it to adapt to dynamic environments, resist corrosion, and maintain structural integrity. Its ability to recover its shape after deformation makes it ideal for minimally invasive procedures.
How does surface quality impact nitinol tubing performance?
A smooth surface finish reduces friction and minimizes tissue damage during implantation. It also lowers the risk of bacterial adhesion and enhances corrosion resistance. Advanced finishing techniques, such as electropolishing, ensure the tubing meets medical-grade standards.
Why is dimensional precision critical in nitinol tubing?
Dimensional precision ensures compatibility with medical devices and reliable performance. Accurate diameter and wall thickness measurements prevent device failure. Tight tolerances and consistent manufacturing standards guarantee the tubing meets the rigorous demands of medical applications.
What certifications should a nitinol tubing supplier have?
Suppliers should hold certifications like ISO 13485 and FDA clearance. These certifications confirm adherence to quality management systems and regulatory standards. They also demonstrate the supplier's commitment to producing safe and effective medical-grade materials.
How do coatings enhance nitinol tubing?
Specialized coatings improve biocompatibility, reduce wear, and increase corrosion resistance. For example, hydrophilic coatings create a lubricious surface for smoother device insertion. Drug-eluting coatings release therapeutic agents, while antimicrobial coatings prevent bacterial growth, enhancing overall performance.
See Also
The Manufacturing Process of Nitinol Tubing for Medicine
Choosing the Right Nitinol Tubing Supplier for You
A Comprehensive Guide to Selecting Nitinol Tubing

