Essential Guide to ASTM F2063 Standards for Nitinol Tubing

ASTM F2063 defines the essential standards for nitinol tubing, setting strict requirements for chemical composition, mechanical properties, and overall performance. Engineers and manufacturers rely on these standards to guarantee the safety and quality of nitinol tubing in medical and engineering fields. Nitinol, with its unique shape memory and superelastic characteristics, plays a vital role in medical devices where reliability and safety cannot be compromised. The importance of astm standards becomes clear in applications like surgical implants and ASTM F2063-Compliant Nitinol Tubing for Thrombectomy Applications, where tubing must meet the highest quality benchmarks. Medical professionals trust nitinol astm f2063 tubing for consistent results, biocompatibility, and proven safety. Rigorous testing and adherence to f2063 standards ensure that every piece of nitinol tubing delivers optimal performance, supporting the growing demand for advanced medical technologies and emphasizing the ongoing importance of astm standards in safety and quality.
Key Takeaways
ASTM F2063 sets strict quality and safety standards for nitinol tubing used in medical devices, ensuring reliable performance and biocompatibility.
Nitinol tubing must meet precise chemical composition and mechanical property requirements to deliver its unique shape memory and superelastic effects.
Certified nitinol tubing undergoes rigorous testing and quality control during manufacturing to guarantee consistent durability and corrosion resistance.
Medical devices using ASTM F2063-compliant nitinol tubing benefit from improved flexibility, fatigue resistance, and long-term safety for patients.
Always verify ASTM F2063 certification before using nitinol tubing to ensure compliance with regulatory standards and optimal device performance.
ASTM F2063 Overview
Standard Definition
ASTM F2063 sets the industry benchmark for nitinol tubing. This standard, developed by ASTM International, defines the chemical, mechanical, and performance requirements for nickel-titanium alloys. Nitinol ASTM F2063 tubing must meet strict guidelines to ensure safety and reliability in medical environments. The standard covers everything from alloy composition to mechanical properties, ensuring that each batch of tubing performs consistently. ASTM standards for nitinol require rigorous testing and documentation. Manufacturers must verify that tubing meets all criteria before it enters medical applications.
ASTM F2063 ensures that nitinol tubing achieves the necessary quality for use in critical medical devices.
Aspect | Details |
|---|---|
ASTM F2063 Definition | Standard specification by ASTM for nickel-titanium shape memory alloy defining chemical, mechanical, and performance requirements. |
Medical Grade | Complies with biocompatibility and mechanical integrity standards for healthcare applications. |
Manufacturing Process | Includes precise alloy composition, thermal processing, hot and cold working to tailor properties. |
Mechanical Properties | Shape memory effect and superelasticity are key features enabling device functionality and reliability. |
Application Focus | Primarily used in medical devices such as stents, guidewires, orthopedic implants due to unique properties. |
Quality Assurance | Strict testing protocols for tensile strength, elasticity, resistivity, and density ensure batch consistency. |
Scope for Nitinol
The scope of ASTM F2063 covers nitinol tubing used in a wide range of medical and engineering applications. The standard specifies the required nickel content, which must fall between 54.5% and 57%. This precise composition allows nitinol tubing to exhibit both shape memory and superelasticity. ASTM standards for nitinol also limit non-metallic inclusions and require corrosion resistance, which is vital for long-term medical use. The standard outlines the manufacturing process, including vacuum melting, forging, and heat treatment, to achieve the desired properties. Nitinol ASTM F2063 tubing must pass tests for density, resistivity, and thermal conductivity.
Property | Value / Range | Description / Relevance |
|---|---|---|
Density | 6.45 - 6.75 g/cm³ | Lightweight yet strong, ideal for medical devices. |
Resistivity | 80 - 100 micro-ohm-centimeters | Maintains shape memory behavior and conductivity. |
Thermal Conductivity | 10 - 20 W/mK | Supports temperature-dependent phase changes. |
Shape Memory Effect | Present | Returns to original shape when heated, essential for medical applications. |
Superelasticity | Present | Allows large reversible deformations, increasing flexibility and durability. |
Quality Control | Rigorous testing | Ensures compliance with ASTM F2063 for consistent performance and safety. |
Medical Applications
Nitinol ASTM F2063 tubing plays a crucial role in modern medical devices. Manufacturers use this tubing in transcatheter heart valves, vascular stents, and endovascular grafts. The unique properties of nitinol, such as superelasticity and shape memory, allow devices to adapt to the human body and maintain performance over millions of cycles. Peer-reviewed studies show that controlling inclusion size in nitinol tubing can double the fatigue strain limit, which increases device durability. Medical professionals rely on nitinol ASTM F2063 tubing for its biocompatibility and resistance to corrosion. The standard requires that tubing pass tests for fatigue life, tensile strength, and surface finish. These requirements ensure that nitinol tubing remains safe and effective in demanding medical environments.
Nitinol ASTM F2063 tubing supports innovation in medical technology, providing the foundation for safer, more reliable devices.
Material Properties
Chemical Composition
Nitinol shape memory alloy stands out due to its precise chemical composition, which directly impacts its performance in medical and engineering applications. ASTM F2063 defines strict limits for each element in the alloy to ensure high quality astm f2063 tubing. The nickel content must range from 54.5% to 57.0%, while titanium makes up the balance. Other elements, such as carbon, cobalt, copper, chromium, hydrogen, iron, niobium, nitrogen, and oxygen, have maximum allowable percentages to maintain the alloy’s purity and reliability.
Element | Composition Range or Max (%) |
|---|---|
Nickel | 54.5 - 57.0 |
Carbon | ≤ 0.040 |
Cobalt | ≤ 0.050 |
Copper | ≤ 0.010 |
Chromium | ≤ 0.010 |
Hydrogen | ≤ 0.005 |
Iron | ≤ 0.050 |
Niobium | ≤ 0.025 |
Nitrogen | ≤ 0.005 |
Oxygen | ≤ 0.040 |
Titanium | Balance |
Testing laboratories conduct chemical composition inspections at every stage—raw material, semi-finished, and finished tubing. Personnel monitor and record each process step, ensuring full compliance with nitinol astm f2063 requirements. A robust quality tracing mechanism tracks product history, guaranteeing consistent quality for every batch. Studies comparing nitinol wires produced by different melting practices confirm that controlling impurities like oxygen and carbon is essential. Lower impurity levels lead to fewer inclusions, which directly improves fatigue life and reliability. Metallographic and SEM analyses reveal that smaller inclusions, such as titanium carbides and intermetallic oxides, correlate with better fatigue performance. These findings reinforce the importance of chemical purity and microcleanliness in nitinol shape memory alloy for medical-grade tubing.
Note: High quality astm f2063 nitinol tubing must meet all chemical composition limits to ensure optimal shape memory effect and superelasticity.
Shape Memory and Superelasticity
The unique properties of nitinol shape memory alloy—shape memory effect and superelasticity—make it indispensable for advanced medical devices. The shape memory effect allows the alloy to return to its original shape after deformation when exposed to a specific temperature. Superelasticity enables the tubing to undergo significant reversible deformation without permanent damage, which is critical for devices that experience repeated mechanical stress.
Fatigue life data from ultra-high-cycle fatigue studies demonstrate the endurance of nitinol tubing under cyclic loading.
Bending fatigue behavior improves with higher microstructural purity, especially in VAR-melted superelastic nitinol.
Statistical analysis links microcleanliness and inclusion size to fatigue resistance, confirming the role of alloy purity.
Accelerated fatigue testing on stent-like specimens provides measurable fatigue life under variable strain amplitudes.
Modeling and numerical simulations validate the shape memory effect and cyclic performance under finite strains.
ASTM F2633 standard for wrought seamless nitinol tubing ensures consistent mechanical properties supporting superelasticity.
Studies show that impurity levels directly affect fatigue life, with cleaner nitinol shape memory alloy exhibiting superior durability.
Crystallographic and X-ray tomography studies provide microstructural evidence for superelastic deformation mechanisms.
Fatigue-crack growth experiments and full-field fracture measurements confirm nitinol’s resistance to fatigue failure.
Cyclic fatigue testing demonstrates the tubing’s endurance under repeated mechanical stress, supporting superelastic performance.
Mechanical testing of nitinol shape memory alloy tubes under tension, compression, and bending quantifies their shape memory and superelastic response. High compressive pre-strains reduce bending fatigue life, defining the operational limits for medical devices. Studies on nitinol stents deployed in anatomically accurate artery models further quantify fatigue performance under realistic service conditions. These results highlight the critical role of high quality astm f2063 nitinol tubing in ensuring device safety and longevity.
Nitinol ASTM F2063 Requirements
Nitinol astm f2063 sets comprehensive requirements for tubing used in medical and engineering applications. The standard specifies not only chemical composition but also mechanical properties, transformation temperatures, surface condition, and dimensional tolerances. Manufacturers must document tensile strength, elongation, and transformation temperatures for each batch. The Af temperature at delivery status must be specified to ensure proper phase transformation behavior, which is essential for the shape memory effect.
Compliance Metric / Quality Parameter | Description / Value |
|---|---|
Shape Recovery Strain | ~4.16%, enabling the alloy to return to its original shape |
Superelastic Strain | ~7%, allowing significant deformation without permanent damage |
Chemical Composition | ~55% nickel, 45% titanium, strict impurity control |
Control of Impurities | Limits on oxygen and carbon to prevent oxides/carbides |
Mechanical Properties | Tensile strength and elongation for super-elastic and cold-worked states |
Transformation Temperatures | Af temperature specified at delivery |
Surface Condition | Defined at delivery for quality assurance |
Dimensional Tolerances | OD and ID with precise tolerances |
Heat Treatment Status | Super-elastic or cold worked, affecting mechanical behavior |
Testing Procedures | Tensile and thermal analysis with documented results |
Biocompatibility Testing | Per ISO 10993, including cytotoxicity, sensitization, irritation, systemic toxicity, genotoxicity |
Certification | Must confirm compliance with ASTM F2063 and regulatory standards |
Testing laboratories perform rigorous quality control assessments, including tensile strength tests and thermal analyses, to verify compliance with nitinol astm f2063. Accredited labs conduct these tests on samples from each production batch, ensuring impartiality and accuracy. Manufacturers implement chemical composition inspections at multiple stages, along with strict process controls and quality tracing mechanisms, to guarantee that only high quality astm f2063 tubing reaches the market.
Peer-reviewed studies confirm that nitinol shape memory alloy produced under astm f2063 standards exhibits low impurity levels and superior fatigue performance. For example, commercial-scale low-oxygen grade Ni50.8Ti49.2, processed by vacuum-arc remelting, achieves oxygen content below 60 ppm and carbon below 20 ppm. These levels result in minimal inclusions and excellent mechanical properties of nitinol, supporting the demands of critical medical applications.
Tip: Always verify that nitinol tubing is certified to astm f2063 before use in medical devices to ensure compliance, safety, and long-term reliability.
Manufacturing and Quality
Processing and Annealing
Manufacturers of certified nitinol tubing follow strict procedures to meet medical grade astm f2063 requirements. The process begins with vacuum-induction melting or vacuum arc re-melting. These methods help control impurities and ensure high quality astm f2063 nitinol. After melting, the material undergoes forging and rolling. Tube drawing uses optimized die sizes, mandrel materials, and lubricants. This step controls the outer diameter-to-wall thickness ratio, which is critical for medical applications. Annealing follows, which recrystallizes the nitinol matrix and stabilizes its superelastic properties. Continuous drawing operations can affect tube uniformity. Non-linear drawing may introduce stress and strain gradients, so manufacturers monitor these factors closely. Although the standard does not specify exact annealing data, it emphasizes process control to achieve consistent quality and compliance.
Testing and Inspection
Testing and inspection protocols for medical grade astm f2063 nitinol tubing ensure long-term reliability. Laboratories perform corrosion resistance tests, including long-term immersion studies. These tests confirm that nitinol tubing resists pitting corrosion under physiological conditions. Fatigue testing measures how cyclic loading affects material integrity. Studies show that fatigue life varies with test speed and environment. High cycle fatigue testing, sometimes exceeding 10^7 cycles, is essential for medical devices. Surface inspections check for geometric consistency and structural integrity. Laboratory corrosion protocols and finite element analysis models verify that tubing meets astm f2063 certification standards. These steps guarantee that only high quality astm f2063 tubing reaches the market.
Certification for Medical Grade ASTM F2063
Certification for medical grade astm f2063 nitinol tubing involves rigorous documentation and third-party verification. Manufacturers must provide detailed records of chemical composition, mechanical properties, and transformation temperatures. Accredited laboratories conduct independent tests to confirm compliance with astm f2063 certification requirements. Each batch of certified nitinol tubing receives a certificate of analysis. This document proves that the tubing meets all regulatory compliance standards and medical grade astm f2063 criteria. Nitinol tubing supplier companies must maintain traceability for every lot. Regulatory agencies require proof of astm f2063 certification before approving medical devices. Consistent certification practices ensure that medical professionals receive only high quality astm f2063 nitinol tubing for critical applications.
Applications in Medical Devices
ASTM F2063-Compliant Nitinol Tubing for Thrombectomy Applications
ASTM F2063-compliant nitinol tubing for thrombectomy applications sets the standard for performance and safety in neurovascular procedures. Clinical studies show a technical success rate of 97% for nitinol stents in these applications. Physicians achieve complete aneurysm occlusion rates up to 89% within 18 months, demonstrating effective vessel remodeling. Nitinol tubing provides superelasticity and the shape memory effect, allowing precise navigation through complex vascular pathways. This flexibility enhances procedural efficiency, reducing operating times and complication rates by up to 25%. Biocompatibility and durability minimize complications, supporting long-term device reliability. Flow-diverting nitinol stents promote thrombus formation and uniform wall shear stress, aiding vessel healing. ASTM F2063-compliant nitinol tubing for thrombectomy applications ensures quality and compliance for critical medical devices.
High technical success rate (97%) in neurovascular procedures
Up to 89% complete aneurysm occlusion within 18 months
Enhanced flexibility and navigation for complex vessels
Reduced operating times and complication rates by 25%
Long-term reliability and biocompatibility
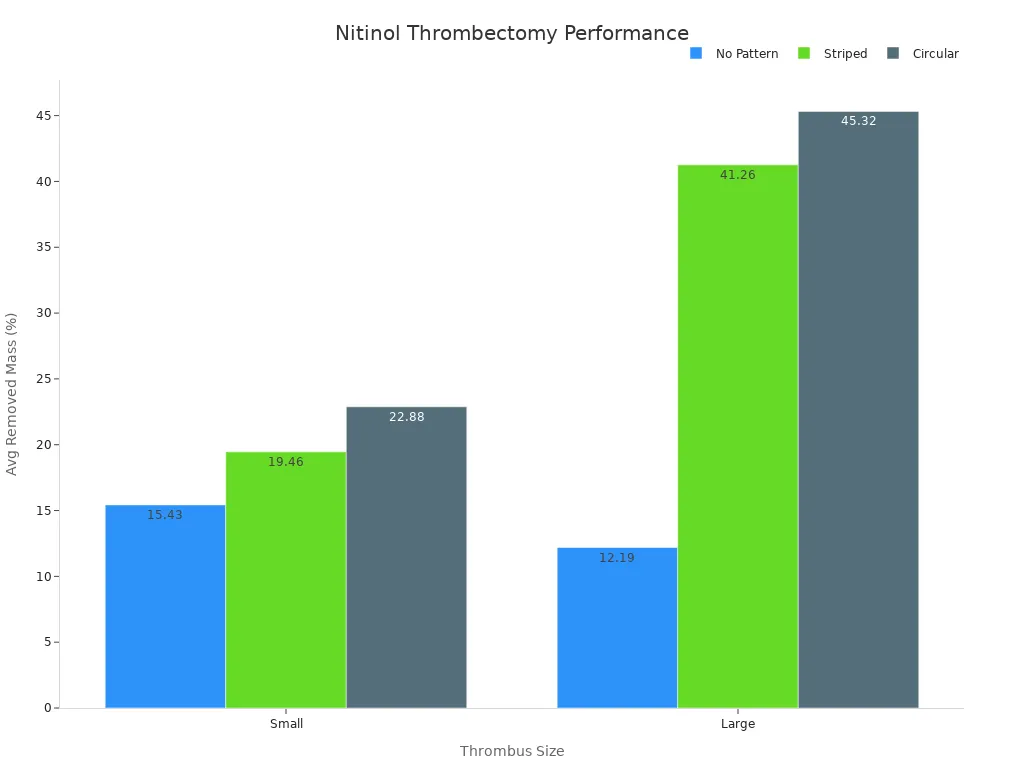
Stent Retriever Type | No Pattern | Striped | Circular |
|---|---|---|---|
Thrombus Size | Small | Large | Small |
Number of Trials | 4 | 4 | 5 |
Avg. Removed Thrombus Mass [%] (X ± SD) | 15.43 ± 8.43 | 12.19 ± 12.00 | 19.46 ± 12.02 |
Statistical Significance | N/A | p < 0.05 | N/A |
Cardiovascular and Orthopedic Uses
Medical grade astm f2063 nitinol tubing supports a wide range of cardiovascular and orthopedic applications. Nitinol’s superelasticity and high tensile strength enable self-expanding stents that adapt to vessel anatomy, improving deployment and patient comfort. Devices made from nitinol shape memory alloy withstand repeated mechanical stress, ensuring durability and reducing failure risk. Orthopedic implants use certified nitinol tubing to conform to patient anatomy, provide stable fixation, and promote faster healing. The flexibility and miniaturization of nitinol devices allow for minimally invasive procedures, leading to quicker recovery and fewer complications. Medical professionals rely on medical grade astm f2063 tubing for its corrosion resistance and long-term safety.
Self-expanding stents adapt to vessel anatomy
High fatigue resistance ensures device durability
Corrosion resistance maintains device integrity
Orthopedic implants promote stable fixation and healing
Minimally invasive procedures support faster recovery
Biocompatibility and Safety
Medical grade astm f2063 nitinol tubing undergoes rigorous biocompatibility testing according to ISO 10993 standards. These tests include cytotoxicity, sensitization, irritation, systemic toxicity, and genotoxicity assessments. Clinical studies confirm excellent biocompatibility with minimal adverse reactions over more than 10 years. Medical-grade tubing ensures patient safety and supports long-term implantation in medical devices. Compliance with astm f2063 standards guarantees that nitinol shape memory alloy meets strict safety requirements. Manufacturers document every step to maintain traceability and uphold safety in all applications.
Note: Always verify compliance and certification for medical grade astm f2063 tubing to ensure optimal safety and device performance.
Updates and Trends
Standard Revisions
Recent revisions to astm f2063 reflect the industry's commitment to safety and performance in nitinol tubing. The standards now require even tighter control over the nickel-titanium ratio in the alloy, ensuring consistent transformation temperatures and mechanical properties. Manufacturers must document every step, from vacuum arc remelting to final inspection, to guarantee purity and uniformity. The latest astm f2063 standards also specify advanced measurement techniques, such as laser micrometers and ultrasonic gauges, for precise dimensional control. These updates help maintain outer diameter tolerances as tight as ±0.005 mm for tubing below 0.3 mm.
Passivation processes, especially water-boiling passivation, have become standard practice. This method reduces nickel ion release from nitinol tubing to 75 ppb, improving biocompatibility and corrosion resistance for medical applications.
The table below summarizes key quantitative requirements in the latest astm f2063 standards:
Aspect/Parameter | Quantitative Data/Description |
|---|---|
Nickel-Titanium Ratio | Precisely controlled for stable alloy properties |
Transformation Temperature | Maintained at 20 ± 3°C |
Shape Recovery Strain | 4.16% |
Superelastic Strain | Up to 7% |
Endurance Requirement | Over 600 million cardiac cycles without failure |
Nickel Release after Passivation | 75 ppb |
Dimensional Tolerances | ±0.005 mm for tubing below 0.3 mm |
Innovations in Nitinol
Advancements in nitinol alloy technology continue to drive improvements in astm f2063-compliant tubing. Manufacturers now use microstructure control to limit non-metallic inclusions to within 5.4 μm, with an area ratio of just 0.5%. This innovation increases the reliability of the alloy in demanding medical applications. Mechanical stability testing confirms that nitinol tubing maintains performance after 20 cycles of 6% strain recovery.
Surface finishing techniques, such as electropolishing and chemical etching, achieve roughness values (Ra) of 0.1 μm or less, which enhances corrosion resistance.
Heat treatments now enable load-bearing capacities above 1400 N, supporting more robust device designs.
Hybrid materials, combining fiber metal laminates with pre-strained nitinol wires, show up to 60% improvement in onset strain for delamination and 34% for transverse crack strain.
Researchers have also optimized the placement of shape memory alloy wires within composite laminates to maximize impact absorption. These innovations ensure that astm f2063 tubing meets the highest standards for flexibility, durability, and safety in cardiovascular and other medical applications.
ASTM F2063 certification remains essential for ensuring the safety, quality, and operational reliability of nitinol tubing in medical devices. Manufacturers achieve consistent certification by following strict alloy processing and surface finishing protocols, which double fatigue resistance and minimize nickel release.
Fatigue life testing confirms that ASTM F2063 certification supports robust tubing performance across diverse environments, with minimal variation in fatigue strength.
High-purity nitinol alloy and optimized processing, as defined by ASTM F2063 certification, directly improve device durability and safety.
Growth Factor | Explanation |
|---|---|
Increasing neurological disorders | Drives demand for certified nitinol tubing in medical devices. |
Technological advancements | Require ASTM F2063 certification for new medical devices. |
More surgical procedures | Increase the need for quality-certified tubing. |
Ongoing awareness of ASTM F2063 certification updates ensures that manufacturers deliver high-quality tubing for advanced medical devices.
FAQ
What is the importance of ASTM F2063 certification for nitinol tubing?
ASTM F2063 certification confirms that nitinol tubing meets strict quality and safety standards. Medical device manufacturers rely on this certification to ensure tubing performs reliably in critical applications. Regulatory agencies require certification before approving devices for clinical use.
How does certification impact the manufacturing process?
Certification requires manufacturers to document every step, from raw material selection to final inspection. They must use accredited laboratories for testing and maintain traceability for each batch. Certification ensures consistent quality and supports regulatory compliance for medical devices.
Who verifies the certification of nitinol tubing?
Independent, accredited laboratories perform third-party testing and inspection. These organizations issue certification documents after confirming compliance with ASTM F2063 requirements. Manufacturers must provide these documents to customers and regulatory bodies as proof of quality.
Can a device be marketed without ASTM F2063 certification?
Medical devices that lack ASTM F2063 certification may not receive regulatory approval. Certification demonstrates that the tubing meets essential safety and performance criteria. Without certification, manufacturers risk product recalls, legal issues, and loss of market access.
How often should manufacturers renew certification for nitinol tubing?
Manufacturers must renew certification with every production batch. Each batch requires new testing and documentation to confirm ongoing compliance. Regular certification updates help maintain high standards and support continuous improvement in manufacturing processes.
Tip: Always request up-to-date certification documents from your nitinol tubing supplier to ensure product quality and regulatory compliance.

